Session Information
Date: Monday, November 9, 2020
Title: SLE – Treatment Poster II
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Hydroxychloroquine (HCQ) and chloroquine (CQ) are antimalarial (AM) medications prescribed for a variety of rheumatic diseases, including systemic lupus erythematosus (SLE). Many patients will remain on these medications for years, and possibly lifelong. HCQ and CQ are associated with irreversible vision loss secondary to retinal toxicity. The prevalence of AM-induced retinopathy varies between studies and few studies have compared prevalence rates between rheumatologic conditions. The purpose of this study was to describe the pattern and risk factors for AM-associated retinopathy.
Methods: A retrospective chart review was conducted at an urban academic Canadian centre after obtaining ethics approval. Each patient was classified as SLE, based on ACR criteria, or non-SLE. The minimum duration of AM therapy was three months. AM-induced retinopathy was classified as possible or definite, which was determined based on characteristic visual field loss, abnormal retinal imaging and eye specialist’s opinion. Univariate and multivariate regression analyses were performed to determine factors associated with definite AM-induced retinopathy. Sensitivity analyses included stratification of analysis by diagnosis and by HCQ versus CQ.
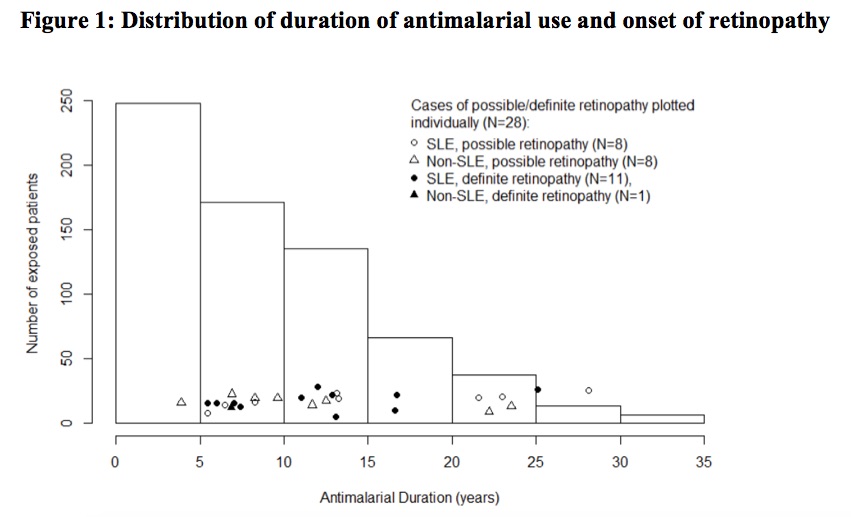
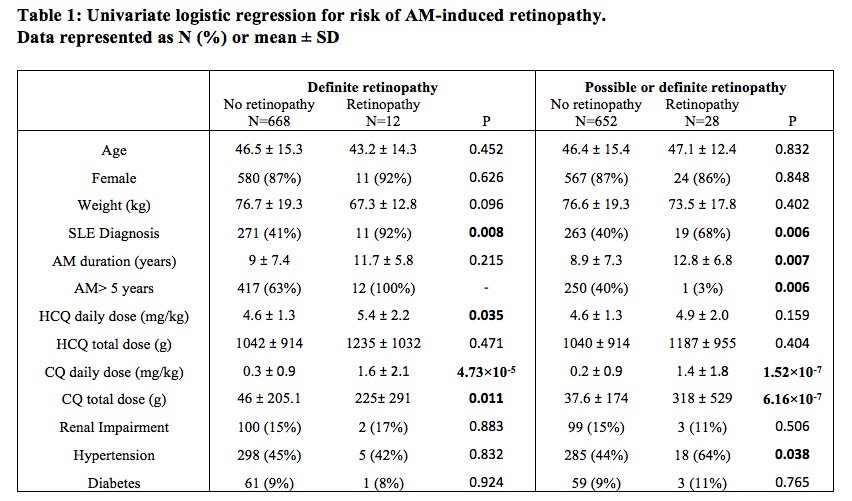
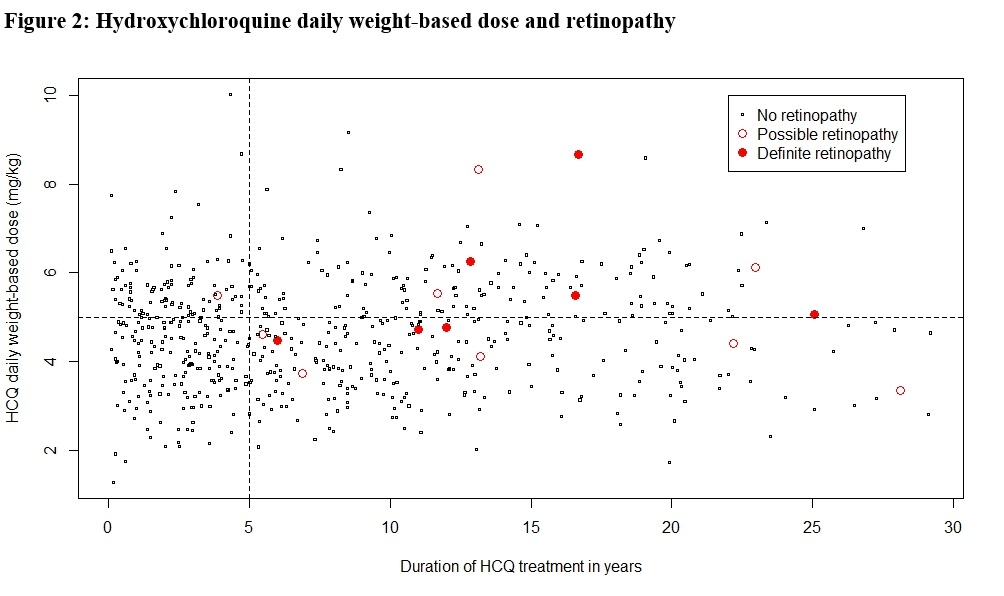
Results: A total of 680 patient charts were reviewed of whom 282 patients had SLE and the remaining had rheumatoid arthritis (N=224), cutaneous lupus (N=41) or other connective tissue diseases (N=133). Ninety percent of patients had a baseline eye examination within the first five years of AM treatment, and 74% had regular follow up exams beyond five years. Definite AM-induced retinal toxicity was observed in 12 patients, 11 of whom had SLE and 7 had been prescribed CQ (Figure 1). The earliest diagnosis of toxicity occurred after 5.4 years of AM therapy, and the prevalence beyond five years was 2.7% but increased over time. In univariate analysis (Table 1), a diagnosis of SLE (P=0.008; OR=16.1; 95% CI=[2.1, 125]), the daily weight-based dose of HCQ (P=0.035; OR=1.5; 95% CI=[1.0, 2.3]), daily weight-based dose of CQ (P< 0.001; OR=1.9; 95% CI=[1.4, 2.6]), and cumulative CQ dosage (P=0.011; OR=5.0; 95% CI=[1.4, 17.3]) were significantly associated with definite AM-induced retinopathy. In multivariate analysis, diagnosis of SLE (P=0.015; OR=13.2; 95% CI=[1.6, 111.3]) and CQ daily weight-based dose (P=0.004; OR=2.0; 95% CI=[1.2, 2.3]) were significantly associated with ocular toxicity after adjusting for CQ/HCQ dosages, age, sex, weight, and renal impairment. When possible retinopathy was included in the univariate analysis, SLE and CQ dosages remained significant (Table 1). Total AM duration and hypertension also had significant associations. Patients prescribed HCQ doses less than the recommended maximum of 5mg/kg/day did develop retinopathy (Figure 2).
Conclusion: The risk of AM-induced retinal toxicity increases after five years of therapy. There may be higher rates of AM-induced retinopathy in SLE patients due to longer duration of treatment, more chloroquine use in this population, and SLE may be an independent risk factor.
To cite this abstract in AMA style:
Cramarossa G, Liu H, Pope J. Risk Factors for Antimalarial-Induced Retinal Toxicity in Systemic Lupus Erythematosus and Other Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/risk-factors-for-antimalarial-induced-retinal-toxicity-in-systemic-lupus-erythematosus-and-other-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-factors-for-antimalarial-induced-retinal-toxicity-in-systemic-lupus-erythematosus-and-other-rheumatic-diseases/