Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Biosimilar use in North America is relatively low, and real-world comparisons of biosimilars and their originator biologics are lacking. We assessed risk factors associated with serious infections in new users of originator infliximab or infliximab biosimilar.
Methods: We used MarketScan administrative health data to create a cohort of adult new users of infliximab (originator infliximab or infliximab biosimilar), between Jan.-Dec. 2017. The first infusion was the cohort entry date. A 90-day current exposure period was assigned for every infusion and individuals could contribute person-time through the observation period. We assessed frequency and time to first serious infection, defined as those associated with hospitalization. Crude incidence rates were generated to compare infection risk between originator infliximab and infliximab biosimilar. Cox proportional hazards regression models were adjusted to identify risk factors associated with serious infections: current infliximab therapy (originator or biosimilar), age, sex, prior biologic use, prior and current use of DMARDs and systemic glucocorticoids, past hospitalized infection, age-adjusted Charlson comorbidity index (CCI), and underlying conditions (rheumatoid arthritis, ankylosing spondylitis, psoriasis/psoriatic arthritis, Crohn’s disease, ulcerative colitis).
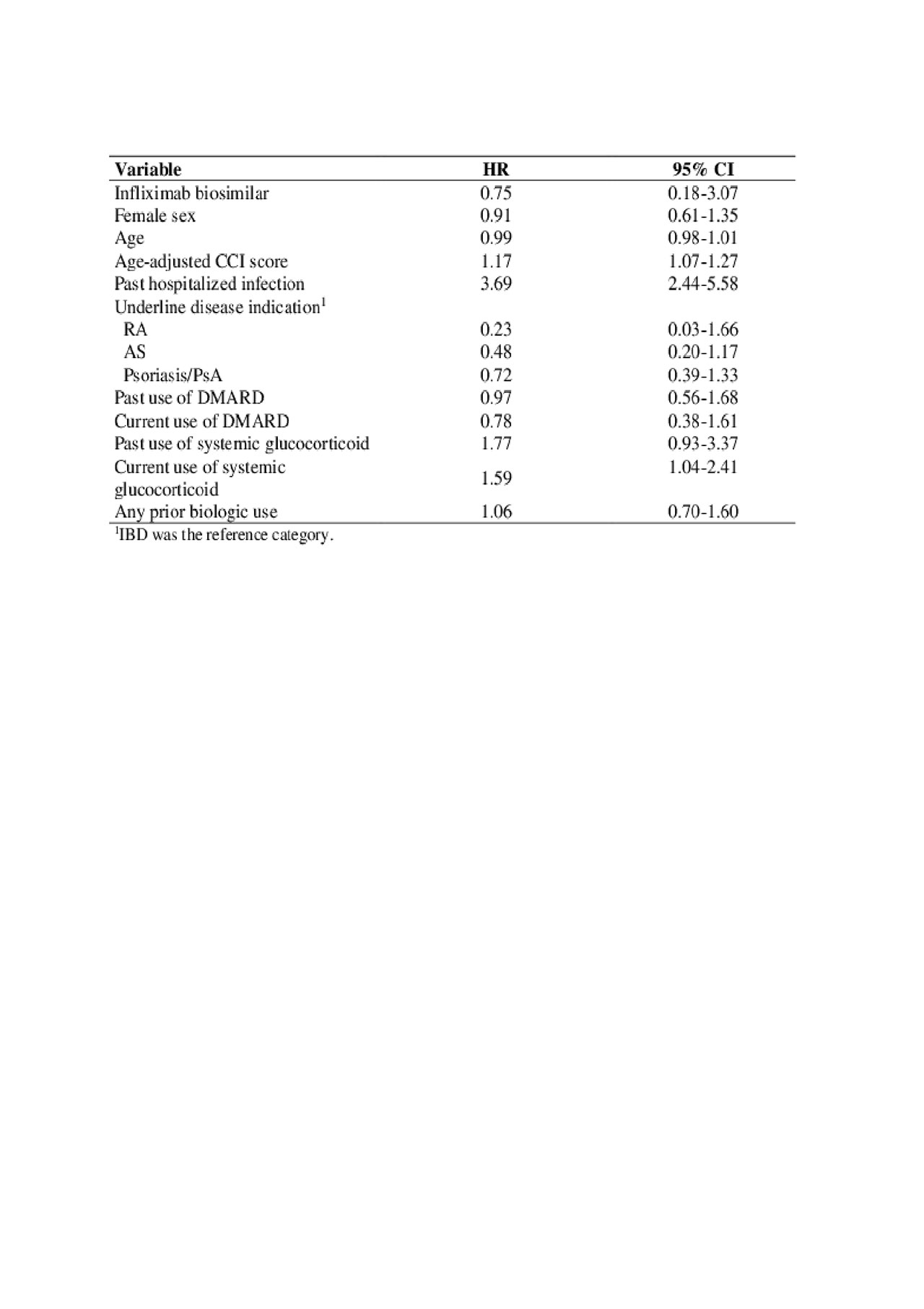
Results: We studied 2676 individuals, including 2584 originator infliximab and 92 infliximab biosimilar. Most (60%) were women and the mean age was 44±15 years. Baseline characteristics (stratified by the initial treatment) are presented in Table 1. We identified 115 hospitalized infections during follow-up. Infection rates were 5.5 (95% confidence interval, CI 1.4-22.1) for current infliximab biosimilar and 8.5 (95% CI 7.0-10.3) for originator infliximab. We were unable to identify an association between infliximab therapy and hospitalized infection (adjusted hazard ratio, HR: 0.75, 95%CI 0.18-3.1). Age-adjusted CCI, past hospitalized infection, and prior and current use of glucocorticoids were associated with risk of hospitalized infection (Table 2).
Conclusion:
We were unable to detect differences in serious infectious between originator infliximab and infliximab biosimilar. High comorbidity score, occurrence of past infections and use of glucocorticoids were associated with increased risk of hospitalized infections. Additional long-term studies would be of additional help in establishing safety profiles.
To cite this abstract in AMA style:
Moura C, Curtis J, Choquette D, Boire G, Bykerk V, Thorne C, Maksymowych W, Lakatos P, Svenson L, Targownik L, Afif W, Bernatsky S. Risk Factors Associated with Serious Infections Among Users of Biosimilar and Originator Infliximab Therapies [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/risk-factors-associated-with-serious-infections-among-users-of-biosimilar-and-originator-infliximab-therapies/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-factors-associated-with-serious-infections-among-users-of-biosimilar-and-originator-infliximab-therapies/