Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Gout flare (GF) prophylaxis in patients (pts) with chronic kidney disease (CKD; estimated glomerular filtration rate [eGFR] <60 ml/min/1.73m2) remains challenging, since prophylaxis with NSAIDs or colchicine may not be advisable in these patients. Three phase 3 trials demonstrated that weekly subcutaneous administration of rilonacept, an IL-1 antagonist, reduced GFs in pts initiating urate-lowering therapy (ULT). This post hoc analysis of the 3 trials evaluated rilonacept in pts stratified by presence and absence of CKD.
Methods: PRESURGE-1 and PRESURGE-2 were similar randomized confirmatory efficacy trials with a 16-week treatment period (~80 pts per group: placebo [Pbo], rilonacept 80mg/week [R80], rilonacept 160mg/week [R160]); RESURGE was a large 16-week randomized safety trial that also assessed GFs. Pts with eGFR <30ml/min/1.73m2 were excluded from these studies. In this analysis, only pts with initial serum urate ≥7.5 mg/dL, ≥2 gout flares in the prior year, and initiating ULT were included. GFs/pt, and % of pts with ≥1 and ≥2 GFs were analyzed stratified by baseline eGFR <60 ml/min/1.73m2 and ≥60 ml/min/1.73m2, and included pts pooled from PRESURGE-1 and PRESURGE-2; RESURGE alone; and pts pooled from all 3 studies. Safety was based on adverse events (AEs) in the overall safety database stratified by eGFR.
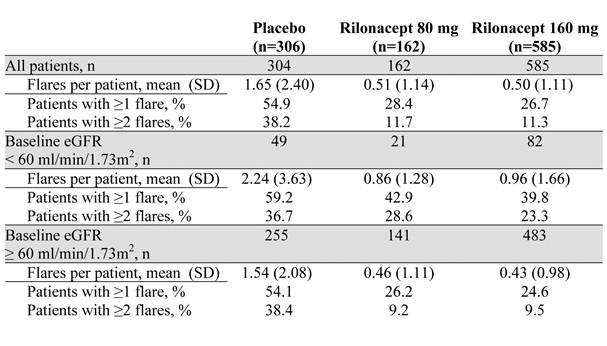
Results: While pooled data from PRESURGE-1 and PRESURGE-2 showed that for pts with CKD, R80 (n=21) and R160 (n=19) reduced GFs/pt by 33% and 53% relative to Pbo (n=26), respectively, the effect on the proportion of pts with GFs was variable, likely due to low pt numbers. Results from the much larger RESURGE study showed that for CKD pts (Pbo, n=23; R160, n=63), GFs/pt were reduced by 65%, from 3.13 (Pbo) to 1.11 (R160), with fewer pts reporting ≥1 GF (41% vs 74%) and ≥2 GFs (24% vs 48%); for pts with eGFR ≥60 (Pbo, n=120; R160, n=339), GFs/pt were reduced by 75%, from 1.78 (Pbo) to 0.45 (R160), with fewer pts on R160 reporting ≥1 GF (25% vs 53%) and ≥2 GFs (10% vs 38%). Pooled data from the 3 studies showed a consistent effect of rilonacept to prevent GFs in pts with CKD across the endpoints (Table). In studies of rilonacept for GF prophylaxis, within each eGFR category the incidence of treatment-emergent AEs and serious AEs, respectively, were similar between treatment groups (eGFR <60: placebo, 64.2% and 8.6%; all rilonacept, 67.6% and 6.0%. eGFR ≥60: placebo, 59.0% and 3.3%; all rilonacept 65.6% and 3.0%), although SAEs were more frequent in the eGFR <60 subgroup.
Conclusion: Rilonacept demonstrated efficacy and tolerability for GF prophylaxis in pts with CKD for whom other agents might be inadvisable.
Disclosure:
R. Terkeltaub,
Regeneron,
5,
BioCryst,
5,
Ardea,
5,
Novartis Pharmaceutical Corporation,
5,
Metabolex,
5,
Takeda,
5,
Savient,
5,
Isis,
5,
UCB,
5,
URL,
5,
Chugai,
5;
R. R. Evans,
Regeneron,
3,
Regeneron,
1;
S. P. Weinstein,
Regeneron,
1,
Regeneron,
3;
R. Wu,
Regeneron,
1,
Regeneron,
3;
H. R. Schumacher,
Takeda, Wyeth,
2,
Regeneron, Novartis, Ardea, Pfizer, Savient, Metabolex,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rilonacept-for-gout-flare-prophylaxis-in-patients-with-chronic-kidney-disease-analysis-of-3-clinical-trials/