Session Information
Date: Saturday, November 6, 2021
Title: Abstracts: Immunological Complications of Therapy (0437–0440)
Session Type: Abstract Session
Session Time: 9:15AM-9:30AM
Background/Purpose: Immune checkpoint inhibitors (ICIs) provide effective treatment for many cancers but, presumably due to persistent activation of the immune system, they cause a variety of immune-related adverse events (irAEs) in almost every organ. Rheumatological manifestations have been reported in ~5-10% of patients treated with ICIs. We aimed to analyze the rheumatological irAEs (rh-irAEs) reported to the FDA Adverse Event Reporting System (FAERS) from October 2012 through March 2021.
Methods: From October 1st, 2012, to March 31st, 2021, we studied all case reports found on the FAERS database when any of the following seven FDA-approved ICIs, nivolumab, pembrolizumab, ipilimumab, atezolizumab, durvalumab, avelumab, or cemiplimab, was the primary suspect of the reported adverse events (AEs).
Of the 6,090 different AEs reported for the ICIs, we selected 186 irAEs, which were rheumatological manifestations, or were associated with rheumatological conditions. We calculated the frequencies of rh-irAEs for each ICI. RStudio v1.4.1106 was used for general data analysis and the R package openEBGM v0.8.3 was used for the calculation of disproportionality scores such as the Empirical Bayes Geometric Mean (EBGM) with its 90% two-sided credibility interval, frequently used in safety signal detection models. Drug-event combinations with an EBGM 5% lower limit credibility interval ≥ 1 were considered significant.
Results: During the study period, 90,974 individual case safety reports (ICSR) included 236,239 AEs with one ICI as the primary suspect of the AE. The highest frequency of AEs was reported for nivolumab (49.6%), followed by pembrolizumab (23.4%), ipilimumab (12.6%), atezolizumab (8.6%), durvalumab (4.6%), avelumab (0.9%), and cemiplimab (0.3%). The AEs were more frequent in males (62.7%) than in females (37.3%). Of the total ICSRs, 84.2% were expedited because they reported serious, unexpected irAEs.
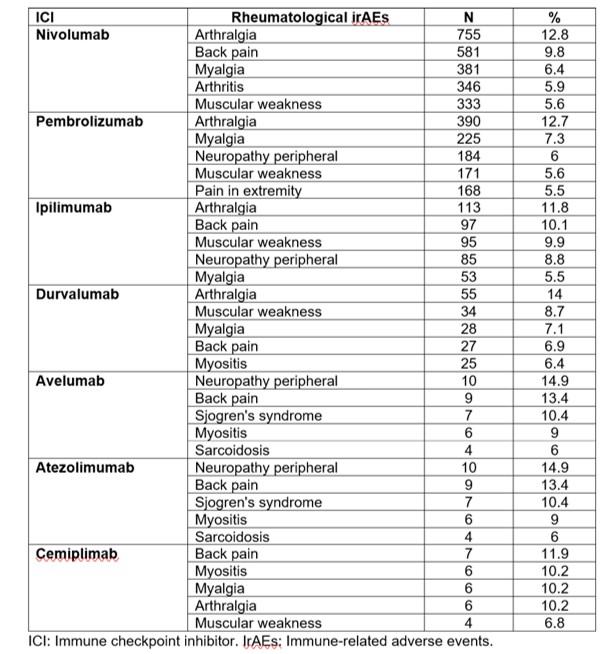
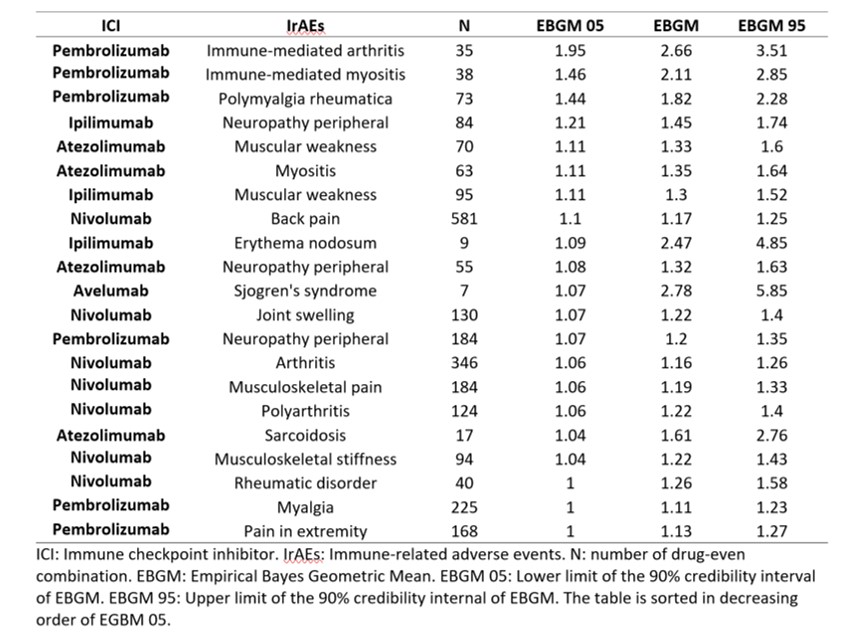
Rh-irAEs were 11,203 (4.7%) out of the 236,239 AEs reported. These rh-irAEs were reported in 3,898 (4.3%) out of the 90,974 ICSRs. For the ICSRs containing rh-irAEs, 78.5% were expedited. Unspecific complains, such as arthralgia, myalgia or muscle weakness were among the most frequent rh-irAEs. Avelumab and atezolizumab were associated with Sjogren’s syndrome and sarcoidosis. Durvalumab, avelumab, atezolimumab and cemiplimab were all associated with myositis (Table 1). Twenty-one drug-event combinations were significant for EBGM (Table 2). Of those, nivolumab and pembrolizumab were the two most frequent ICI, with 7 and 6 significant drug-event combinations, respectively.
Conclusion: Approximately 5% of the reported ICIs-associated AEs were rh-irAEs. The most frequent complaints were unspecific, such as arthralgia, myalgia, or muscle weakness. Arthritis, myositis, Sjogren’s syndrome, and sarcoidosis were also relatively frequent. The improved understanding of the mechanism of action of the ICIs and the characteristics of the rh-irAEs may help to elucidate the pathogenesis of the autoimmune disorders that they trigger.
To cite this abstract in AMA style:
Rodriguez-Pla A. Rheumatological Immune-Related Adverse Events of Immune Checkpoint Inhibitors Based on the FDA Adverse Event Reporting System [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/rheumatological-immune-related-adverse-events-of-immune-checkpoint-inhibitors-based-on-the-fda-adverse-event-reporting-system/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatological-immune-related-adverse-events-of-immune-checkpoint-inhibitors-based-on-the-fda-adverse-event-reporting-system/