Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Upadacitinib 15 mg QD (UPA), an oral JAK inhibitor, showed greater efficacy compared to adalimumab (ADA) in patients (pts) with active rheumatoid arthritis (RA) on background MTX at wk 12 of the SELECT-COMPARE phase 3 study1. A subset of pts switched treatment (ADA to UPA [23.5%] and UPA to ADA [19.2%]) due to non-response (NR) to the initial therapy. A higher proportion of pts rescued from ADA to UPA achieved CDAI ≤10 (LDA) post-switch (47%) vs pts rescued from UPA to ADA through 24 wks (36%)2.
We explore the biological mechanisms associated with the differential clinical response in RA pts who switched treatment due to NR to initial ADA or UPA therapy in comparison to pts who continued treatment based on response (R) to the initial therapy.
Methods: We included a random subset of pts (100 pts each from the originally randomized treatment groups [UPA, n=651; ADA, n=327]). NR was defined as < 20% improvement in TJC or SJC at wks 14–24 compared to baseline (BL) and R as achieving these improvements. The levels of 181 inflammation-related protein biomarkers (BM) were analyzed using the Olink platform at BL, wks 2, 8, and 26. Contrasts between relative BM levels were assessed using a repeated measure mixed linear model, and correlations between BM levels and DAS28-CRP were computed using Pearson’s method.
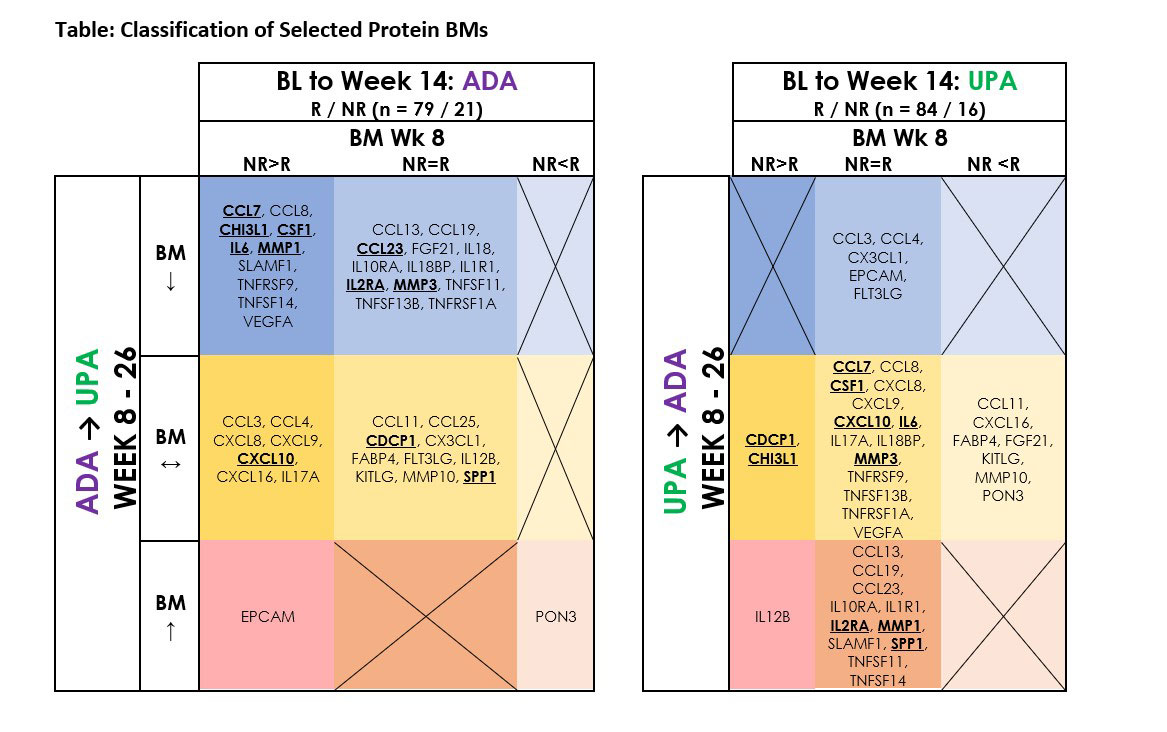
Results: In this patient subset, 40% of ADA NR who switched to UPA achieved CDAI LDA at wk 26 vs 17% of UPA NR who switched to ADA. Proteomics analysis identified 42 BMs that showed significant differential modulation between R and NR prior to switch (BL to wk 8) or after switch (wks 8–26) (Table). Ten BMs (CCL7, CCL8, CHI3L1, CSF1, IL6, MMP1, SLAMF1, TNFRSF9, TNFSF14, and VEGFA) were differentially modulated between ADA R and NR and were significantly decreased after switching to UPA (wk 26). The levels of these 10 BMs were similar between UPA R and NR through wk 8, except for CHI3L1, and MMP1, SLAMF1, and TNFSF14 increased after switching to ADA (wk 26). Another group of 13 BMs (CCL13, CCL19, CCL23, FGF21, IL18, IL10RA, IL18BP, IL1R1, IL2RA, MMP3, TNFSF11, TNFSF13B, TNFRSF1A) had similar levels between ADA R and NR through wk 8 and were significantly decreased after switching to UPA (wk 26). The levels of these 13 BMs were similar between UPA R and NR, except for FGF21, and the levels of CCL13, CCL19, CCL23, IL10RA, IL1R1, IL2RA, and TNFSF11 increased after switching to ADA (wk 26). Notably, the BL levels of CCL7, CCL23, CHI3L1, CSF1, IL2RA, IL6, MMP1, MMP3, and VEGFA were correlated with DAS28-CRP at BL, suggesting a potential relationship of these BMs in the pathobiology of RA. Among the remaining BMs, the levels of CCL3, CCL4, CX3CL1, EPCAM, and FLT3LG were similar between UPA R and NR and decreased after switching to ADA (wk 26).
Conclusion: We propose that switching to UPA from ADA NR leads to more favorable clinical improvement that is paralleled by a more profound immune modulation than switching to ADA from UPA NR, consistent with the broader mechanistic inhibition achieved by JAK-associated signaling pathway inhibition compared with discrete TNF-signaling inhibition.
References
- Fleischmann R. et al. Arthritis Rheumatol 2019; 71:1788–1800.
- Fleischmann R. et al. Ann Rheum Dis 2021; 80:432–9.
To cite this abstract in AMA style:
Sornasse T, Cai F, Camp H, Song I, McInnes I. Rheumatoid Arthritis Patients Who Switched Treatment from Adalimumab to Upadacitinib Demonstrate a Robust Reduction of Inflammation-related Biomarkers: Proteomics Analysis from the SELECT-COMPARE Phase 3 Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/rheumatoid-arthritis-patients-who-switched-treatment-from-adalimumab-to-upadacitinib-demonstrate-a-robust-reduction-of-inflammation-related-biomarkers-proteomics-analysis-from-the-select-compare-phas/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatoid-arthritis-patients-who-switched-treatment-from-adalimumab-to-upadacitinib-demonstrate-a-robust-reduction-of-inflammation-related-biomarkers-proteomics-analysis-from-the-select-compare-phas/