Session Information
Date: Monday, October 22, 2018
Title: 4M102 ACR Abstract: Misc Rheum & Inflam DZ I: DADA2, Cardiac Sarcoid,Cancer Immunotherapy(1911–1916)
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Rheumatologists are increasingly called upon to manage the autoimmune side effects of immune-checkpoint inhibitors (ICIs). However, these new entities are poorly understood and treatment often relies upon retrospective studies and expert opinion. The objective of this study is to describe the prevalence, clinical presentation, and management of a large cohort of patients with rheumatic immune-related adverse effects (Rh-irAEs) from immune checkpoint inhibitor therapy.
Methods: From a database of all patients who received any immune-checkpoint inhibitor at the Mayo Clinic Rochester, Minnesota campus between January 1st, 2011 and March 1st, 2018, we identified those with Rh-irAEs using diagnostic codes, search terms, and manual chart review.
Results: Of the 1,293 patients who received any checkpoint inhibitor, 43 were clinically diagnosed with Rh-irAEs. Eighteen patients with Rh-irAEs who received ICI therapy elsewhere were also analyzed. Clinical syndromes included inflammatory arthritis (IA) (n=37, prevalence 2%), myopathy (n=10, 0.8%), and connective tissue disease (CTD) (n=14, 0.7%). IA was most commonly polyarticular and 28 patients (76%) required glucocorticoids. Mean treatment duration was 20 weeks (SD 23 weeks). Seven of these patients (19%) also received disease-modifying drugs and 3 patients (8%) required discontinuation of ICI therapy. Myopathy was treated with glucocorticoids in all cases for a mean duration of 15 weeks (SD 17 weeks) and led to two deaths and permanent ICI discontinuation in 9 patients (90%). CTD included cases of sicca syndrome, systemic sclerosis, and vasculitis. Nine of these patients (64%) were treated with glucocorticoids for a mean duration of 31 weeks (SD 30 weeks) and 3 patients (21%) had complete resolution of their Rh-irAE symptoms.
Conclusion: This study represents the largest cohort of Rh-irAEs to date. Most patients required long courses of treatment with only a minority achieving complete symptom resolution. Prospective, multicenter studies are necessary to determine the optimal management of these emerging disorders and further define immunologic phenotypes.
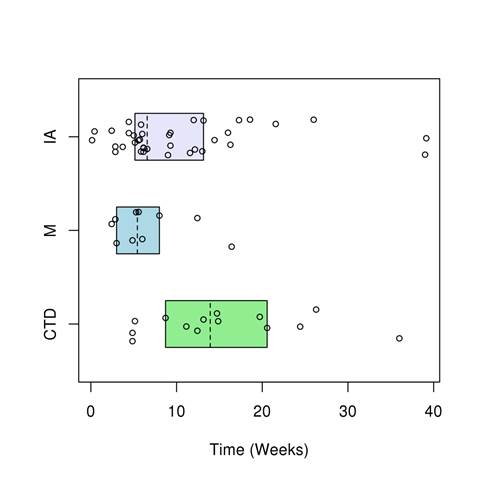
Figure 1. Time of Rh-irAE symptom onset after starting ICI therapy.
Abbreviations: IA; inflammatory arthritis, M; myopathy, CTD; connective tissue disease
Table 1. Inflammatory arthritis clinical features.
|
Characteristic |
Value* |
|
Patients
|
37 |
|
Age, mean (St. Dev.) |
58.9 (16.8) |
|
Prevalence |
2% |
|
Polyarticular |
25 (68%) |
|
Oligoarticular |
12 (32%) |
|
Sedimentation rate, mean (SD) |
43.8 (23.1) |
|
C-reactive protein, mean (SD) |
51.4 (52.7) |
|
Positive RF or ACPA |
3 (8%) |
|
Pre-existing inflammatory arthritis |
3 (8%) |
|
Pre-existing degenerative joint disease |
27 (73%) |
|
Treatment |
|
|
Prednisone only
|
21 (57%) |
|
Prednisone plus disease modifying agent
|
7 (19%) |
|
NSAIDs and/or intraarticular glucocorticoids only |
8 (22%) |
|
Starting prednisone dose, mg/day, mean (SD) |
33.6 (20.5) |
|
Weeks on prednisone, mean (SD)
|
20 (23.4) |
|
Complete resolution after treatment
|
17 (46%) |
|
Partial resolution after treatment
|
20 (54%) |
|
ICI discontinued due to irAE
|
3 (8%) |
*Values in table are mean (Standard deviation) or n (%).
Abbreviations: SD; standard deviation, ICI; immune-checkpoint inhibitor, Rh-irAEs; rheumatic immune-related adverse effects (Rh-irAEs)
To cite this abstract in AMA style:
Richter M, Crowson CS, Kottschade L, Finnes H, Markovic SN, Thanarajasingam U. Rheumatic Syndromes Associated with Immune-Checkpoint Inhibitors: A Single-Center Cohort of 61 Patients [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/rheumatic-syndromes-associated-with-immune-checkpoint-inhibitors-a-single-center-cohort-of-61-patients/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatic-syndromes-associated-with-immune-checkpoint-inhibitors-a-single-center-cohort-of-61-patients/