Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: In May 2025, ACR released updated recommendations for the management of LN2. Notably, since the previous guidelines update in 2012, the FDA has approved belimumab and voclosporin for the treatment of LN.
Methods: 1,017 moderate-to-severe (M/S) adult SLE patient records were collected in collaboration with 176 US rheumatologists via an online survey platform from January through March 2025. This analysis focuses on a subset of 226 SLE patients with biopsy-proven ISN Class III, IV, or V LN disease. These patients also had to exhibit active, new-onset, or flaring renal disease, defined as an LN diagnosis within the past five years, a flare within the past six months, or active renal involvement at their most recent rheumatology visit.
Results: ACR strongly recommends that patients with Class III, IV, or V LN be treated with hydroxychloroquine (HCQ), unless contraindicated. This retroactive chart analysis finds that 88.1% of these patients are currently prescribed HCQ.Second, ACR recommends use of RAAS-I therapy for Class III, IV, or V LN patients with any level of proteinuria in their urine. Our analysis shows that 69.3% of these patients are on RAAS-I therapy, most commonly ACE inhibitors such as lisinopril and enalapril. Third, ACR recommends that oral steroids be tapered to at least 5mg per day within six months of initiating the treatment for Class III, IV, or V LN patients. Furthermore, daily steroid dosages should not exceed 40mg/day. Our analysis shows that 50.4% of patients are currently receiving steroid treatment, with none prescribed more than 40 mg per day. Notably, over half (54.5%) of patients who have been on oral steroids for at least six months are taking doses higher than 5mg daily. Only 51.5% of patients who are on oral steroid dosages exceeding 5mg per day are currently on a regimen consisting of belimumab or a calcineurin inhibitor (CNI), which have been demonstrated to be effective steroid-sparing agents.3,4 Fourth, for patients with Class III or IV ± V LN, ACR recommends a triple therapy regimen of steroids, MMF or cyclophosphamide (CYC), and either a CNI or belimumab. Among the 193 patients meeting these criteria, 19.6% are currently prescribed MMF and steroids in combination with a CNI or belimumab. Furthermore, 23.3% of patients are prescribed MMF and belimumab or a CNI without steroids.
Conclusion: Currently, rheumatologists frequently prescribe HCQ and RAAS-I therapies to their LN patients, in line with the updated ACR guidelines. However, many patients remain on high-dose steroids after six months of treatment. Nearly half of those on high-dose steroids are not prescribed a CNI or belimumab, suggesting an opportunity to reduce steroid use through broader use of these drugs. Additionally, only a minority of qualified Class III or IV ± V patients are on a double therapy regimen (MMF or CYC + belimumab or a CNI ± steroids), underscoring the potential impact guideline adoption could have on the management of this disease.
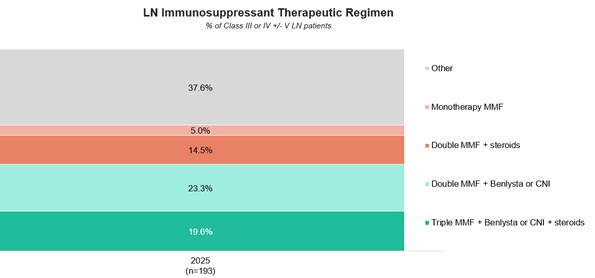 LN Immunosuppressant Therapeutic Regimen
LN Immunosuppressant Therapeutic Regimen
To cite this abstract in AMA style:
May S, Price L, Rex R. Retrospective Chart Audit Reveals Gaps in Steroid Tapering and Use of ACR Guideline-Recommended Regimens in LN [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/retrospective-chart-audit-reveals-gaps-in-steroid-tapering-and-use-of-acr-guideline-recommended-regimens-in-ln/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/retrospective-chart-audit-reveals-gaps-in-steroid-tapering-and-use-of-acr-guideline-recommended-regimens-in-ln/

.jpg)