Background/Purpose:
Retrospective studies have demonstrated that repeat rituximab treatment may be effective in re-inducing remission in relapsing ANCA-associated vasculitis. We analyzed data from the Rituximab in ANCA-associated vasculitis (RAVE) trial in order to determine the safety and efficacy of a second course of rituximab for relapsing granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA).
Methods:
Randomized controlled trial comparing rituximab (RTX, n=99) to cyclophosphamide (CYC) followed by azathioprine (AZA, n=98) for remission induction. Patients who suffered a severe disease flare (BVAS/WG > 3 or one major BVAS/WG item) between 6 and 18 months were eligible for open label RTX (OLR) (375mg/m2 once weekly times four).
Results:
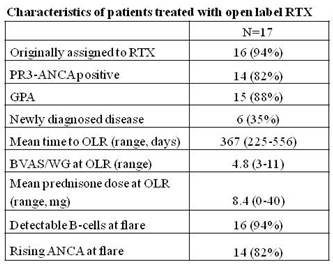
17 patients received two courses of RTX. Baseline characteristics are presented in the table.
After retreatment, patients were followed for an average of 301 days (range 35-427). Treatment with OLR led to remission (BVAS/WG=0) in 15 of 17 patients (88%) by an average of 55 days (range 1-181). One patient with diffuse alveolar hemorrhage did not improve and died 7 weeks after the initial flare. Another patient reached a BVAS/WG of 1 before suffering a limited flare at 12 months after OLR.
Six months after OLR, 15 patients (88%) were in remission (BVAS/WG = 0), 8 (47%) had achieved complete response (BVAS/WG = 0 and prednisone ≤ 10mg/day) and 6 patients (35%) were in complete remission (BVAS/WG = 0 and prednisone = 0). After 12 months, 13 patients (76%) had achieved complete responses and 8 (47%) had reached complete remission. There were 4 limited and no severe flares among the 17 patients (BVAS/WG 2.5) over one year of follow up after OLR.
There were a total of 3 severe (grade ≥ 3) adverse events after OLR, including one death (described above), metastatic colon cancer, and severe sinusitis.
Conclusion:
This prospective study indicates that retreatment of GPA or MPA flares with rituximab is effective in re-inducing remission.
Disclosure:
E. Miloslavsky,
Genentech Inc,
9;
U. Specks,
None;
P. A. Merkel,
None;
P. Seo,
None;
R. F. Spiera,
Roche Pharmaceuticals, g,
2;
C. A. Langford,
Bristol-Myers Squibb,
9,
Genentech and Biogen IDEC Inc.,
9;
G. S. Hoffman,
None;
C. G. M. Kallenberg,
Roche,
8;
E. W. St Clair,
None;
N. Tchao,
None;
L. Ding,
None;
D. Ikle,
Rho,
3;
B. Jepson,
Rho,
3;
P. Brunetta,
Genentech Inc,
3;
J. H. Stone,
Genentech and Biogen IDEC Inc.,
2,
Genentech and Biogen IDEC Inc.,
5,
Roche Pharmaceuticals,
2.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/retreatment-with-rituximab-in-the-rituximab-in-anca-associated-vasculitis-rave-trial/