Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose:
Primary SjšgrenÕs Syndrome (pSS) is a chronic autoimmune rheumatic disease causing a wide-range of symptoms including dryness, pain and fatigue. Individual patient experiences of pSS vary resulting in a heterogeneous patient population. We use patient reported symptoms to identify distinct clinical pSS phenotypes and use these to explore underlying biological differences.Methods:
We used Patient Reported Outcome Measures (PROMs) to clinically phenotype 594 patients on the United Kingdom Primary SjšgrenÕs Syndrome Registry. Phenotype patterns were identified using hierarchical cluster analysis of patient reported rating scales for pain, fatigue, dryness, anxiety and depression. Non-parametric analysis of variance was used to evaluate biological differences between these clusters. We then used the same PROMs to phenotype 463 pSS patients from Norwegian and French cohorts, in order to validate these four phenotypes independently.Results:
We identified four phenotypic clusters, which we refer to as Low Symptom Burden (LSB), High Symptom Burden (HSB), Dryness Dominant (DD) and Low Anxiety and Depression (LAD) – with marked differences in health status and quality of life (TTO p <0á0001, EQ-5D VAS p <0á0001). Furthermore, there were significant differences on clinical measures of disease activity (ESSDAI p=0á039), and objective dryness measures (salivary flow p=0á007, SchirmerÕs p=0á014). In addition, there were marked differences in biological parameters (IgG p<0á0001, lymphocytes p=0á0005, ESR p=0á003, IL-17 p=0á0174 and TNFα p=0á0133) between clusters, suggesting possible distinct underlying endotypes. Significant biological and clinical differences in IgG, Lymphocytes, ESR, ESSDAI, and Salivary Flow remained across the four phenotypes in our validation cohorts.Conclusion:
We have identified and independently validated four distinct pSS clinical phenotypes with associated biological differences. There are marked differences in the potential health gains for these four clusters with important implications for clinical management, trial design and therapeutic development for pSS.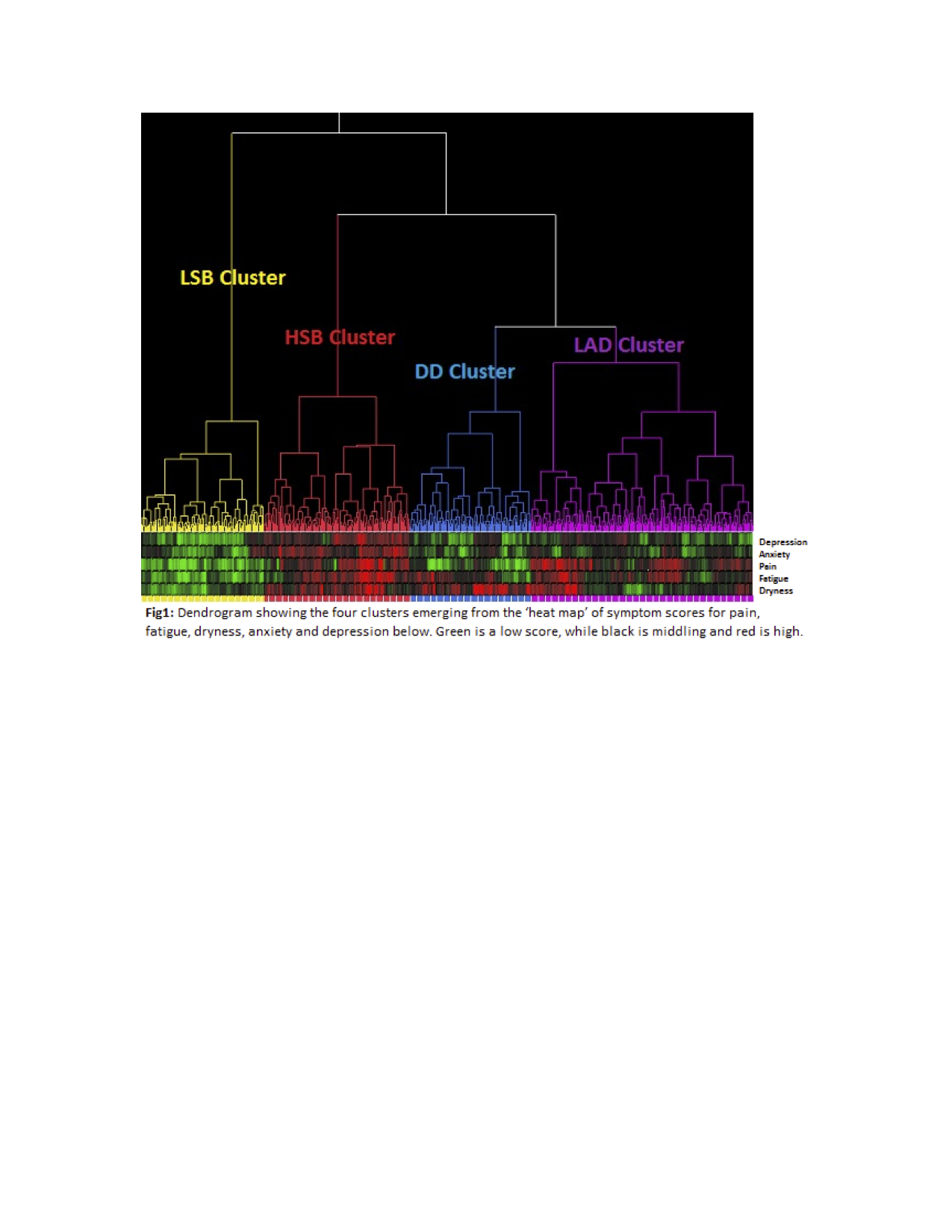
Table 1: Clinical and biological differences across phenotypes. Median values in bold, 25th and 75th centile below, with UKPSSR cohort in white and the two validation cohorts shaded in grey.
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
Disclosure: D. Lendrem, None; N. Howard Tripp, None; X. Mariette, None; S. J. A. Johnsen, None; J. Tarn, None; K. Hackett, None; B. Griffiths, None; S. Mitchell, None; A. Saraux, None; V. Devauchelle, None; K. Norheim, None; J. D. Isaacs, None; P. McMeekin, None; S. Bowman, None; R. Omdal, None; J. E. Gottenberg, None; W. F. Ng, None.
To cite this abstract in AMA style:
Lendrem D, Howard Tripp N, Mariette X, Johnsen SJA, Tarn J, Hackett K, Griffiths B, Mitchell S, Saraux A, Devauchelle V, Norheim K, Isaacs JD, McMeekin P, Bowman S, Omdal R, Gottenberg JE, Ng WF. Rethinking Primary Sjögren’s Syndrome: Stratification By Clinical Phenotypes to Improve Understanding of Disease Pathogenesis, Trial Design, Clinical Management and Prospective Health Gains? [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/rethinking-primary-sjogrens-syndrome-stratification-by-clinical-phenotypes-to-improve-understanding-of-disease-pathogenesis-trial-design-clinical-management-and-prospective-health-gains/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rethinking-primary-sjogrens-syndrome-stratification-by-clinical-phenotypes-to-improve-understanding-of-disease-pathogenesis-trial-design-clinical-management-and-prospective-health-gains/
