Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Ixekizumab, a monoclonal antibody that neutralizes IL-17A with high affinity and specificity, has been evaluated in patients (pts) with moderate to severe rheumatoid arthritis (RA) who were naïve to biologic therapy (bDMARD-naïve) or were inadequate responders to TNF inhibitors (TNF-IR) in a Phase 2 study. The primary endpoint at 12 weeks was achieved, and significant improvements versus placebo (PB) were observed in both populations1. The long-term safety and efficacy after 64 weeks of ixekizumab treatment in the two populations were evaluated in an optional open-label extension (OLE) of the study.
Methods: In this randomized, double-blind study, PB or ixekizumab was administered subcutaneously (SC) to 260 bDMARD-naïve pts (ixekizumab 3, 10, 30, 80, or 180 mg) and 188 TNF-IR pts (ixekizumab 80 or 180 mg) at Weeks 0, 1, 2, 4, 6, 8, and 10 receiving concomitant conventional DMARD therapy (Part A). After a treatment hiatus between Weeks 10 to 16, 232 bDMARD-naïve and 158 TNF-IR pts elected to participate in the OLE period, receiving ixekizumab 160 mg SC at Weeks 16, 18 and 20 and then every 4 weeks (Q4W) through Week 64 (Part B).
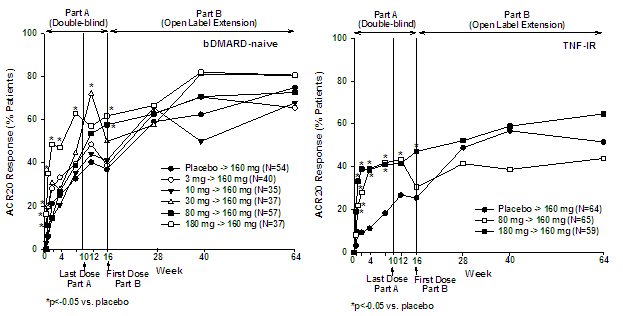
Results: A total of 202 bDMARD-naïve (87%) and 99 TNF-IR (62%) pts completed the OLE period. ACR20 response rates observed at Week 16 were maintained or improved after switching to 160 mg Q4W through Week 64 (Figure). Similar maintenance or improvement was observed for ACR50 and ACR70 responses as well as for DAS28-CRP in both populations. Among bDMARD-naïve pts with an ACR20 (n=107), ACR50 (n=39), or ACR70 (n=13) response at Week 16, this response was maintained in 89%, 77%, and 69% pts, respectively. Among TNF-IR pts with an ACR20 (n=41), ACR50 (n=18) or ACR70 (n=11) response at Week 16, this response was maintained in 76%, 67%, and 44% pts, respectively. Similar maintenance of response was observed for EULAR responses. For pts on PB in Part A, disease activity decreased in Part B to levels comparable with pts originally assigned to ixekizumab groups in Part A. DAS28<2.6 was achieved in 23% bDMARD-naive (n=46) and 22% TNF-IR pts (n=23) after 64 weeks of ixekizumab treatment. Adverse events (AEs) occurred in 73% (n=283) pts during Part B. Most AEs were mild to moderate in severity and did not lead to study discontinuation. Serious AEs were reported in 9% (n=35) pts including 2% (n=9) cases of serious infections. No mycobacterial or systemic fungal infections were reported.
Conclusion: Clinical improvements observed with ixekizumab treatment in the double-blind phase were maintained or improved in the pts that participated in the open label extension period through Week 64. Ixekizumab was well-tolerated, and there were no unexpected safety findings observed in Part B relative to Part A.
1. Genovese et al. (2012) Ann Rheum Dis 71(Suppl3):59
Disclosure:
M. C. Genovese,
Pharmacyclics,
1,
Astellas,
2,
Astellas,
5,
AstraZeneca,
2,
AstraZeneca,
5,
Amgen,
2,
Amgen,
5,
Lilly,
2,
Lilly,
5,
UCB,
2,
UCB,
5,
BMS,
5,
Sanofi-Aventis Pharmaceutical,
2,
Sanofi-Aventis Pharmaceutical,
5,
Novartis Pharmaceutical Corporation,
2,
Novartis Pharmaceutical Corporation,
5,
Pfizer Inc,
5,
Pfizer Inc,
2,
Merck Human Health,
5,
Boehringer Ingelheim,
5,
Genentech and Biogen IDEC Inc.,
5,
Genentech and Biogen IDEC Inc.,
2;
H. Carlier,
Eli Lilly and Company,
1,
Eli Lilly and Company,
3;
J. Erickson,
Eli Lilly and Company,
1,
Eli Lilly and Company,
3;
D. Braun,
Eli Lilly and Company,
1,
Eli Lilly and Company,
3;
S. Banerjee,
Eli Lilly and Company,
1,
Bristol Myers-Squibb,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/results-of-64-weeks-of-treatment-with-an-anti-il-17-antibody-ixekizumab-in-patients-with-rheumatoid-arthritis-in-a-phase-2-study/