Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Since January 2016, Chilean patients with rheumatoid arthritis (RA), with severe activity despite the use of 3 DMARDs for at least 6 months, have guaranteed access to biologics. In the first year the only first-line option was abatacept, in the second year etanercept and adalimumab were added. Our objetive was to evaluate the efficacy at 6 months of abatacept vs TNF-α blockers as first-line biologic.
Methods: Real life, prospective-cohort, single-center study. Only RA patients with a DAS28 ESR greater than 5.1 or with > 6 painful joints, >3 swollen joints, ESR >30 mm/h, and morning stiffness >45 minutes on 2 evaluations, separated between 30-120 days, were allowed to postulate for biologic treatment. Included patients during 2016 received abatacept as first-line biologic, and since 2017 the first-line biologic was selected by randomizing patients in order of attention to abatacept, etanercept or adalimumab, unless there was a particular condition that made it preferable one over another (latent tuberculosis, intersticial lung disease). Tapering of other DMARDs and prednisone was done depending on the clinician’s judgment. The patients were followed for 6 months, DAS28 ESR change was evaluated and the EULAR response criteria was calculated. HAQ, other medications use changes, and adverse events were evaluated. Comparisons were made between abatacept and TNF-α blockers (etanercept/adalimumab). Multivariate analysis was performed taking into account age, sex, years with symptoms, comorbidities, smoking, BMI, CCP status, DMARDs, corticoids, NSAIDs and tramadol use, and baseline DAS28.
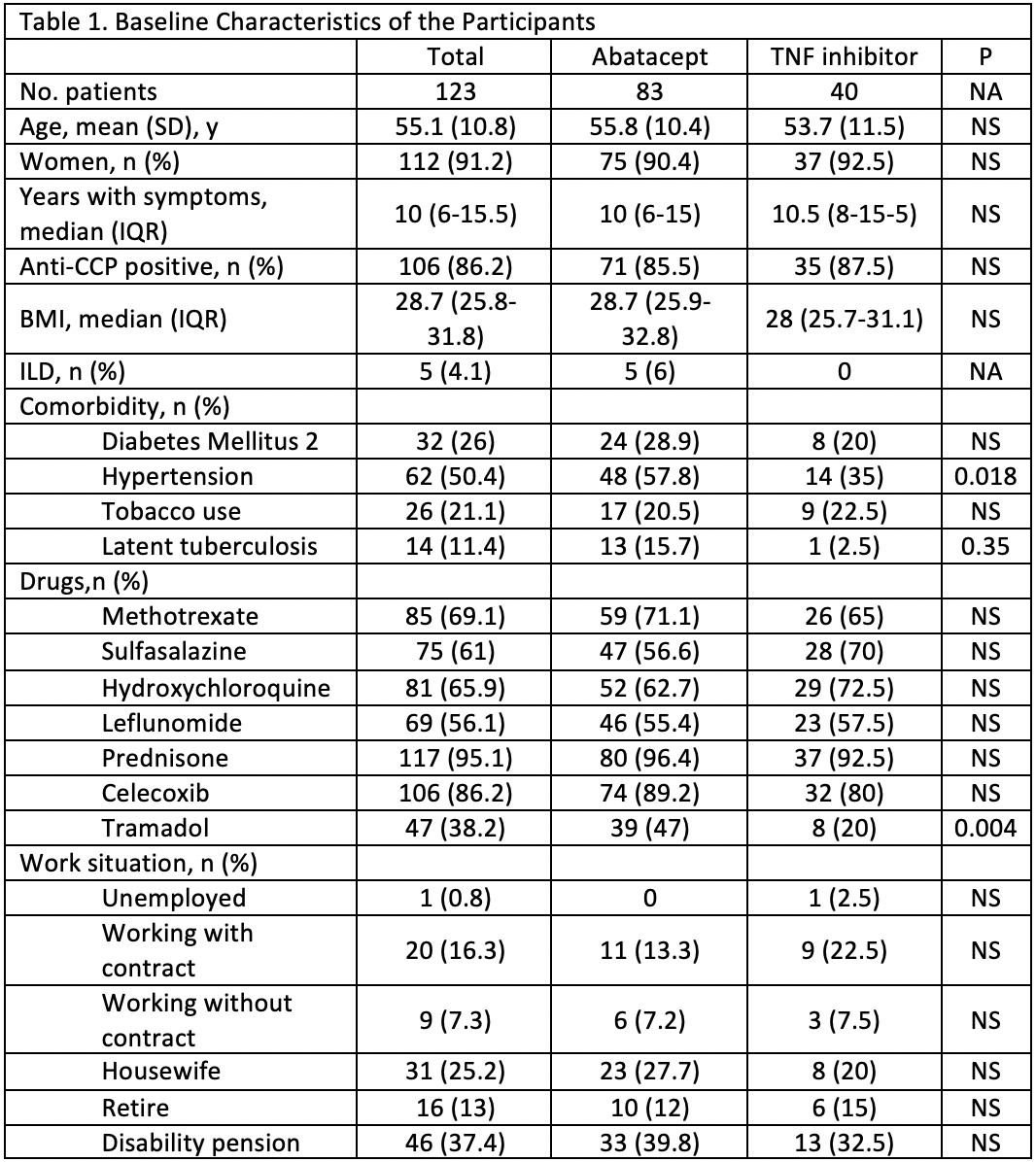
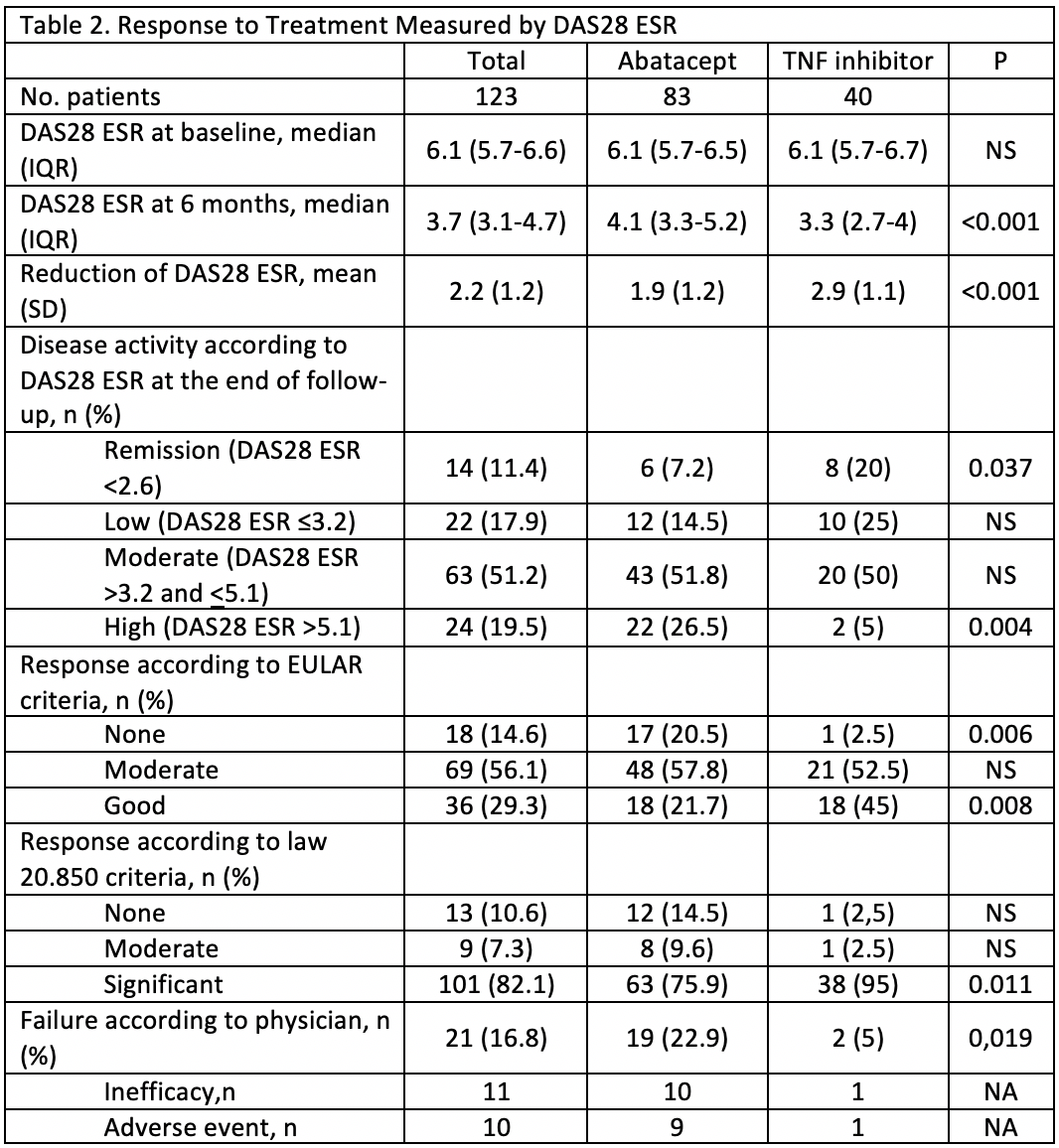
Results: 123 patients were enrolled (table 1 for baseline characteristics). Users of TNF-α blockers had a significantly greater decrease in DAS28 ESR, higher rate of remission, higher rate of good EULAR response, and a lower rate of failure according to the physician (Table 2). These differences maintained statistical significance (p < 0.05) after multivariate analysis. A higher BMI was significantly related, after multivariate analysis, to a lower reduction of DAS28 ESR and lower rate of remission after 6 months. For HAQ there was no difference in the reduction and the result after 6 months of biologic treatment (from 1.9 to 1.5). The median number of DMARDs and the median prednisone dose was reduced without differences between groups. The rate of tramadol use saw a 10.9% reduction in the abatacept group and 2.5% in the TNF-α blocker group (table 3). After multivariate analysis more years with symptoms was related to higher rate of tramadol use after 6 months of biologics. The rate of adverse events (AE) was higher in the TNF-α blocker group (39.1 vs 27.7%) but the rate of serious events was higher in the abatacept group (30.4 vs 22.2% of all AE), although no significant differences were found.
Conclusion: At 6 months, in this real life study, more than 80% of the patients with severe, long standing, DMARDs resistant, RA had at least a moderate response to biologic treatment. This improvement was significantly higher in the TNF-α blocker group. Although the abatacept group had more comorbidities the difference with the TNF-α blocker group remained significant after multivariate analysis.
To cite this abstract in AMA style:
Valenzuela O, Ibanez S, Poblete M, Mardones C, Silva F, Villar M, Mogollones K. Results at 6 Months of Abatacept vs TNF-α Blockers in Patients with Severe, Long-standing, DMARDs Resistant Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/results-at-6-months-of-abatacept-vs-tnf-%ce%b1-blockers-in-patients-with-severe-long-standing-dmards-resistant-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/results-at-6-months-of-abatacept-vs-tnf-%ce%b1-blockers-in-patients-with-severe-long-standing-dmards-resistant-rheumatoid-arthritis/