Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The management of immune thrombocytopenia in childhood-onset systemic lupus erythematosus (cSLE) is not standardized. We examined the efficacy and safety of hydroxychloroquine (HCQ) as monotherapy for thrombocytopenia in cSLE.
Methods: We retrospectively reviewed the medical records of patients who developed thrombocytopenia (platelet count < 100 ×109/L) and were diagnosed with cSLE and followed in the rheumatology clinic at The Hospital for Sick Children (SickKids) between January 2005 and December 2023. In this clinic, structured data, including disease activity (assessed by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)), are prospectively collected at every visit. Definite cSLE was defined by the 2019 EULAR/ACR classification criteria, while patients with incipient cSLE had clinical features of evolving SLE but achieved a EULAR/ACR score below 10. A complete response was defined as a platelet count >100 ×109/L with no bleeding. Partial response was defined as a platelet count >30 ×109/L with at least a 2-fold increase from the lowest count and no bleeding. Descriptive statistics were used to characterize the study groups and outcomes.
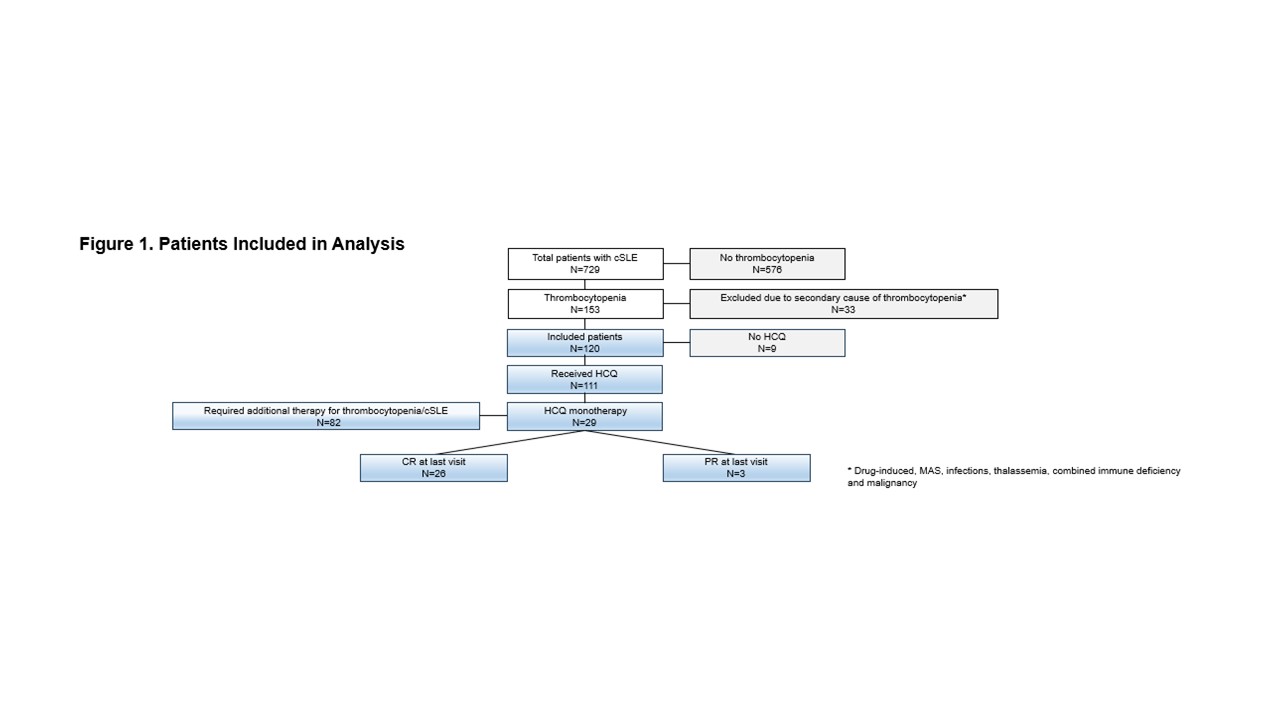
Results: Of the 729 patient records reviewed (68 with incipient lupus and 661 with definite lupus), 153 (21%) patients had thrombocytopenia (Figure 1). Thirty-three patients with thrombocytopenia, secondary to other causes such as medications or infections, were excluded. One hundred twenty patients (79% female) with a median age of 13 years (IQR 10.3 -14.7) and median platelet count of 23 ×109/L (IQR 8.0 – 63.0) at the time of thrombocytopenia diagnosis were included. The median lowest platelet count was 10 ×109/L (IQR 2 – 40). Disease characteristics are detailed in Table 1. Ninety-five (79%) patients had definite, and 25 (21%) had incipient cSLE. The median SLEDAI score at cSLE diagnosis (for definite SLE) was 8 (IQR 5.5 – 16). Eighty-three (69%) of patients were treated with corticosteroids and/or IVIG prior to commencing HCQ, and 45 of them (38%) also required one or more additional treatments for thrombocytopenia (those were azathioprine, mycophenolate mofetil (MMF), eltrombopag, romiplostim, rituximab, or cyclophosphamide). Sixty-six (55%) patients achieved complete or partial responses before initiating HCQ. 111 (93%) patients were treated with HCQ, initiated at a median of 6 months (IQR 2.7 – 12.2) after thrombocytopenia diagnosis at a median platelet count of 77 ×109/L (IQR 32.5 – 198.0). Of those, twenty-nine (24%) patients were treated with HCQ monotherapy (Table 2). All 29 responded; 26 had complete responses after a median follow-up of 52 months (IQR 33 – 75). Of the 111 patients who received HCQ during their disease course, eight experienced side effects, and 6 of them had to discontinue the treatment due to these effects.
Conclusion: While most patients initially needed additional treatments, HCQ monotherapy effectively maintained a partial or complete response in thrombocytopenia for over four years in approximately one-quarter of cSLE patients. Further analysis will identify characteristics that predict a positive response to HCQ monotherapy.
To cite this abstract in AMA style:
Natour H, Goh Y, Dominguez D, Gold N, Ng L, Knight A, Silverman E, Feldman B, Hiraki L, Levy D. Response to Hydroxychloroquine in Immune Thrombocytopenia in Childhood-Onset Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/response-to-hydroxychloroquine-in-immune-thrombocytopenia-in-childhood-onset-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/response-to-hydroxychloroquine-in-immune-thrombocytopenia-in-childhood-onset-systemic-lupus-erythematosus/

.jpg)
.jpg)