Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: To investigate effectiveness and identify predictors of response to belimumab in patients with active SLE in clinical practice setting.
Methods: One hundred eighty eight active SLE (ACR criteria) patients with positive anti-dsDNA antibody and low C3 and/or C4, from 11 Italian prospective lupus cohorts, were treated with belimumab (10 mg/kg day 0, 14, 28 and then every 28 days), as add-on therapy. They were 174 (92.6%) females, mean age 40.7+-10.1 years, mean disease duration 12.7±8.5 years. SLEDAI-2K, anti-dsDNA, C3, C4, prednisone daily dose, DAS-28, 24-hours proteinuria, CLASIa (Cutaneous LE Disease Area and Severity Index Activity) were recorded at baseline, at month 12 and 24. Anti-dsDNA levels were measured by ELISA in 98 patients, IIF in 62 patients, Farr assay in 28 patients. The following variables were included in the univariate and multivariate analysis to determine baseline predictors of response (according to SLE Responder Index-4, SRI-4) at 12 and 24 months: gender, age, disease duration, disease activity pattern (relapsing remitting or chronic active), SLEDAI-2K ≥10, prednisone dose >7,5 mg/day, concomitant immunosuppressants (yes/no), polyarthritis, skin rash, glomerulonephritis (GN), hematologic involvement. Pattern of disease activity was identify as chronic active disease in patients with a SLEDAI-2K ≥2 excluding serology in at least two out of the three annual visits and relapsing-remitting disease in patients with a SLEDAI-2K ≥2 excluding serology in one out of three annual visits. Statistics were performed by pairs T-test, chi-square test and multiple logistic regression analysis using SPSS package (version 22.0).
Results: Mean follow-up period was 17.5+-10.6 months (range 3-36). Active manifestations which required the use of belimumab were polyarthritis in 45.7%, skin rash in 26.1%, GN in 13.8%, and hematologic involvement in 14.4% of cases. Clinical and serological variables at baseline and after 12 and 24 months of follow-up are reported in Table. SRI-4 was achieved by 71.3% and 68.7% of patients at 12 and 24 months, respectively. Baseline independent predictors of response by logistic regression at month 12 were: SLEDAI-2K ≥10 (OR 25.8, 4.19-159.2) and polyarthritis (OR 8.33, 1.88-36.78). Predictors at month 24 were: SLEDAI-2K ≥10 (OR 12.11, 1.63-89.80), polyarthritis (OR 32.56, 2.94-360.56), and prednisone dose >7.5 mg/day (OR 7.88, 1.02-61.48). Discontinuation was observed in 49 patients (26.1%) after 9±7 months of follow-up. Twenty seven patients (55.1%) discontinued for disease activity, 13 (26.5%) for adverse events, 5 (10.2%) for pregnancy. Retention rate was 89.4% at 6 months, 81.4% at 12 months, 76.1% at 18 months, 75.0% at 24 months.
Conclusion: SLEDAI ≥10, polyarthritis and prednisone dose >7,5 mg/day at baseline were the best predictors of response in our cohort of patient with active SLE. 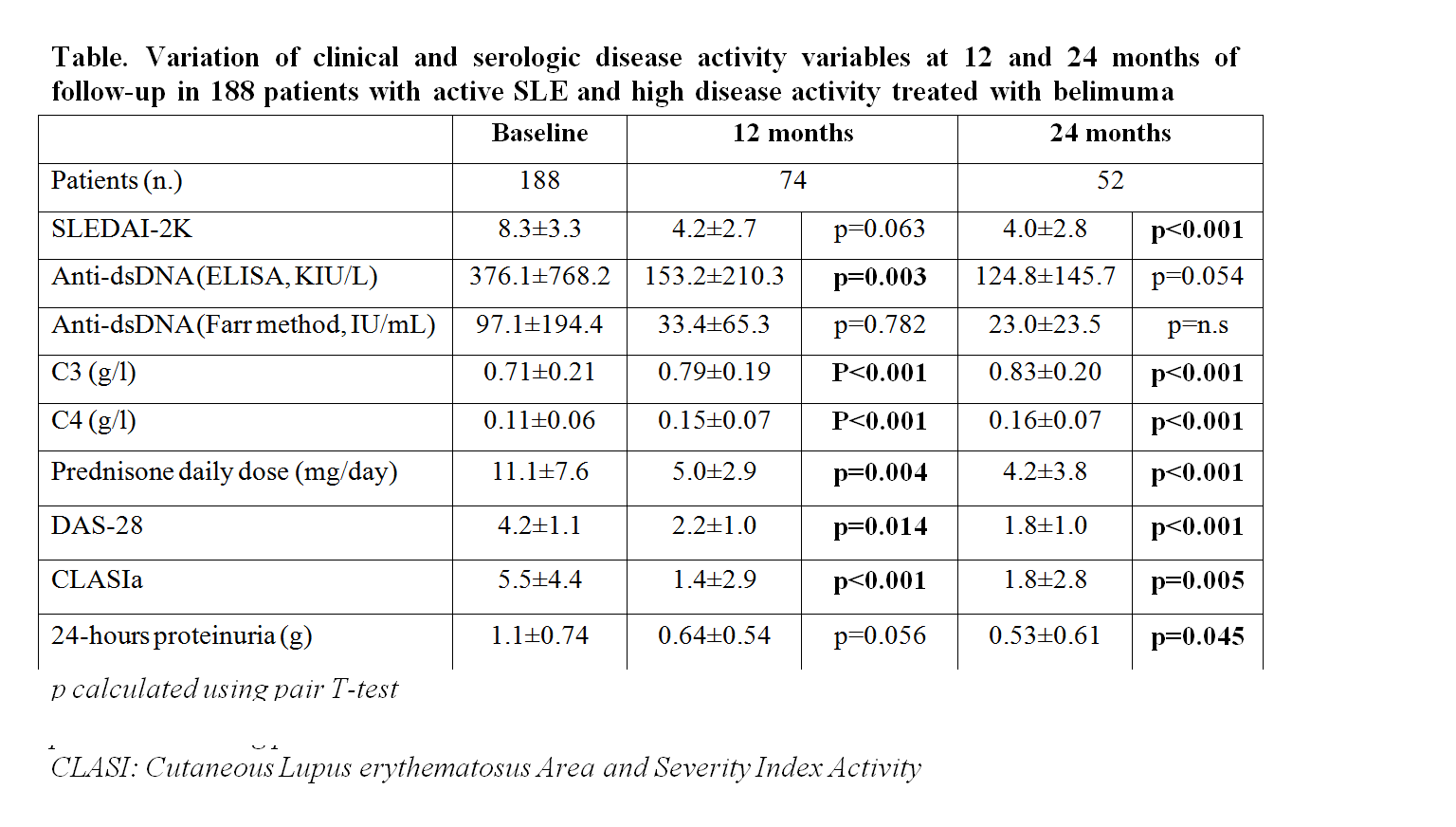 Table. Variation of clinical and serologic disease activity variables at 12 and 24 months of follow-up in 188 patients with active SLE and high disease activity treated with belimuma p calculated using pair T-test CLASI: Cutaneous Lupus erythematosus Area and Severity Index Activity
Table. Variation of clinical and serologic disease activity variables at 12 and 24 months of follow-up in 188 patients with active SLE and high disease activity treated with belimuma p calculated using pair T-test CLASI: Cutaneous Lupus erythematosus Area and Severity Index Activity
To cite this abstract in AMA style:
Iaccarino L, Bettio S, Reggia R, Emmi G, Ceccarelli F, Tani C, Gerosa M, Govoni M, Bortoluzzi A, De Vita S, De Marchi G, De Angelis R, Di Matteo A, Salvarani C, Pazzola G, Bartoloni-Bocci E, Andreoli L, Zen M, Conti F, Mosca M, Meroni PL, Gerli R, Tincani A, Doria A, Emmi L. Response to Belimumab in SLE Patients with High Disease Activity: Data from a Multicentric Clinical-Practice Based Study Cohort [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/response-to-belimumab-in-sle-patients-with-high-disease-activity-data-from-a-multicentric-clinical-practice-based-study-cohort/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/response-to-belimumab-in-sle-patients-with-high-disease-activity-data-from-a-multicentric-clinical-practice-based-study-cohort/
