Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Literature describes a fairly high degree of satisfaction with SLE therapies, despite patients reporting high rates of adverse event (1-2). The objectives of this study were to understand patient satisfaction with their treatment, patient reported treatment burden, desired improvements with therapies, and treatment goal priorities.
Methods: A cross-sectional, non-interventional, online survey was developed based on literature review and expert clinician input. Participants were identified through patient panels and included English-speaking patients >18 years old from the United States who self-reported a SLE diagnosis by a physician. For each class of medications used for the treatment of SLE (e.g. anti-malarials (AM), corticosteroids (CS), immunosuppressants (IS), or biologics, patients indicated their level of satisfaction on a 5-point scale ranging from completely satisfied to very unsatisfied, how burdensome each medication was on a 4-point scale (very burdensome to not at all burdensome), reasons it was considered burdensome, and side effects experienced in the past 3 months. Additionally, patients were asked about their desired improvements with treatment and what they considered to be the most important treatment goals. Descriptive data are presented by means (standard deviation [SD]) for continuous measures, and frequency (n, %) for nominal or ordinal measures.
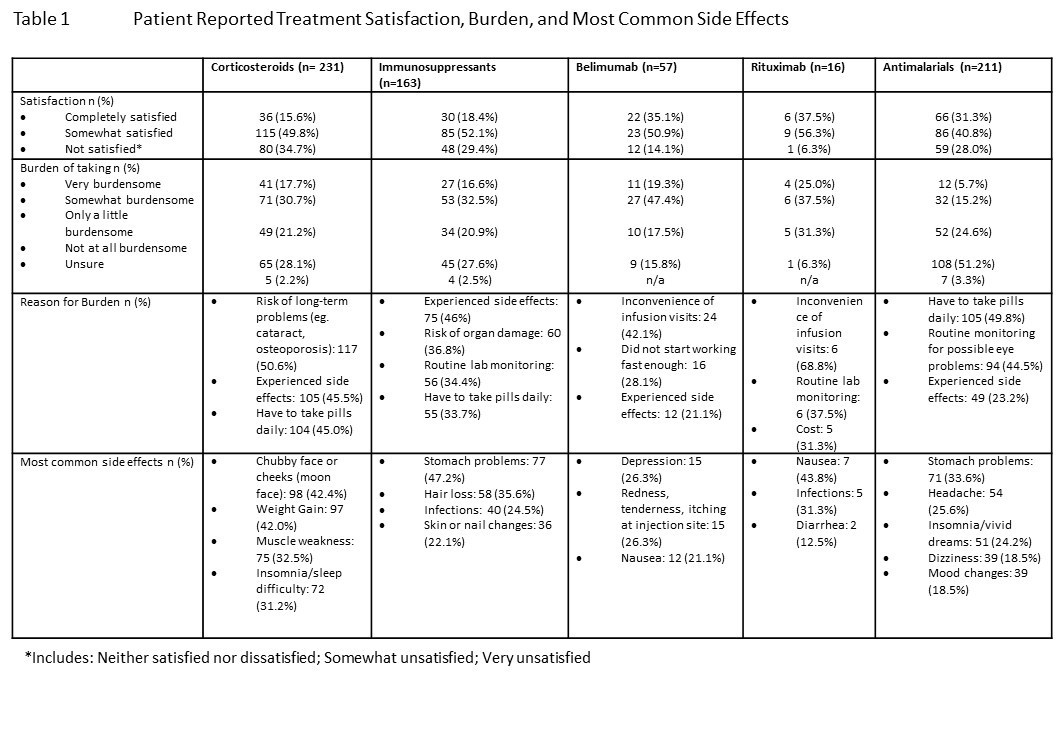
Results: Patients (n=500) were mostly female (75%), Caucasian (76%), with a mean age of 42.6 [12.7] years and mean disease duration of 11.1 [10.6] years. Patient reported current SLE medications included: AM 42%, CS 33%, IS 33%, and biologics 19%. Across medications patients were taking at the time of the survey, 16-38% indicated they were ‘completely satisfied’, and 41-56% indicated they were ‘somewhat satisfied’ (Table 1). CS had the highest percentage of ‘neutral or not satisfied’ responses (35%), while biologics had the largest percentage of ‘satisfied’ patients (86-94%). However, biologics had the highest percentage of patients rating their medication as ‘somewhat’ or ‘very burdensome’ (62-67%). “Reasons for burden” varied by medication, with the most common reported burden being short or long-term side effects, or inconvenient administration. The most common desired improvements to current lupus therapy were keeping lupus disease activity very low (66%), reduction in all symptoms (64%), and reduction in flares (61%). Highest priority lupus treatment goals included reduction in fatigue, pain, and flares. Lower ranked goals were the reduction in use of CS or IS. Over 60% of patients said their physicians did not ask about their most important treatment goals. When they did ask, over 75% of patients felt their goals matched their physician, 16% did not.
Conclusion: Patients report relatively high rates of satisfaction with current therapies, despite identifying high levels of treatment burden. From the patient perspective, reduction in fatigue, pain, and flares are the most important treatment goals.
To cite this abstract in AMA style:
Kamen D, Birt J, Hadi M, Sargalo N, Brookes E, Swinburn P, Hanrahan L, Tse K, Bello N, Griffing K, Silk M, Delbecque L, Askanase A. Residual Treatment Burden, Desired Improvement, and Prioritized Treatment Goals in Systemic Lupus Erythematosus (SLE): Results from the SLE-UPDATE (Understanding Preferences, Disease Activity and Treatment Expectations) Survey in the United States [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/residual-treatment-burden-desired-improvement-and-prioritized-treatment-goals-in-systemic-lupus-erythematosus-sle-results-from-the-sle-update-understanding-preferences-disease-activity-and-trea/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/residual-treatment-burden-desired-improvement-and-prioritized-treatment-goals-in-systemic-lupus-erythematosus-sle-results-from-the-sle-update-understanding-preferences-disease-activity-and-trea/