Session Information
Date: Friday, November 6, 2020
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Discrepancy between patient and provider assessments of disease activity is well described in psoriatic arthritis (PsA) and other inflammatory arthritides. Patients often have low tender (TJC) and swollen joint counts (SJC) but continue to have symptoms they ascribe to their PsA. Limited data exists on the specific residual symptoms that patients describe in discordance with physician assessments of very low disease activity (VLDA). Targeting these additional symptoms could improve patient outcomes without escalation of therapy. The objective of this study was to describe the residual symptoms among patients with low TJC, SJC, and enthesitis counts.
Methods: A cross-sectional observational study was conducted of consecutive PsA patients in the Psoriatic Arthritis Research Consortium (PARC) cohort between 2017-Jan 2020. Participants (pts) who started a new therapy and completed a global assessment of response to treatment, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), and Psoriatic Arthritis Impact of Disease (PsAID) questionnaire were included. We examined the prevalence of BASDAI and PSAID item scores > 4 (score > 4 is considered active disease) among pts with very low physician-assessed disease activity as defined by the provider portions of the VLDA criteria: SJC (0-66) of < 1, TJC (0-68) of < 1, and enthesitis count (Leeds + plantar fascia, 0-8) < 1. Pts global assessment of response to treatment was recorded as improvement, no change, or worsening of symptoms.
Results: 244 PsA pts (mean age 51.1yrs (SD 13.9), 53% female, mean BMI 30.0 (SD 6.6) were identified with the required assessments at baseline and follow up after initiating a new therapy. Therapies initiated (pts could start more than one) included: TNFi (134), IL-17i (50), oral small molecule (88), and other (14). Mean PSAID and BASDAI scores at follow-up were significantly lower for pts in physician-assessed VLDA compared to those not in VLDA (p< 0.001 for almost all comparisons. Table 1); however, 47% of pts in physician-assessed VLDA had at least one residual symptom (score >=4 on BASDAI or PSAID item) compared to 85% not in physician-assessed VLDA using the PsAID. Pts global assessment of response to treatment demonstrated improvement in 42%, no change in 45%, and worsening in 12%. The most common residual symptoms were fatigue (33%), spine pain (30%), peripheral joint pain/swelling (26%), stiffness (23%), disrupted sleep (24%), and tenderness to touch (19%). Many of these symptoms were ascribed to PsA (PsAID items include the phrase “due to my PsA” ). Furthermore, more than 10% of pts in physician-assessed VLDA still reported difficulty with function, work, and skin symptoms. Mean BMI was not significantly different between those in and not in physician-assessed VLDA.
Conclusion: Among PsA patients in VLDA according to physician measures, approximately half continue to have residual symptoms. Further studies are needed to develop comprehensive, evidence-based, patient-focused treatment plans to address these residual symptoms.
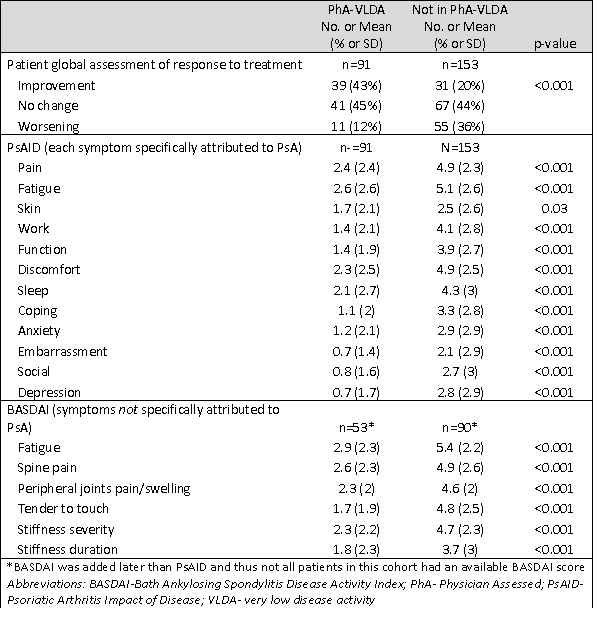 Table 1. Mean differences in patient reported outcomes by physician-assessed very low disease activity
Table 1. Mean differences in patient reported outcomes by physician-assessed very low disease activity
 Figure 1. Residual symptoms among patients in physician-assessed very low disease activity
Figure 1. Residual symptoms among patients in physician-assessed very low disease activity
To cite this abstract in AMA style:
Ogdie A, Reddy S, Craig E, Scher J, Lee Y, Walsh J, Husni M. Residual Symptoms in Patients with PsA Who Are in Very Low Disease Activity According to Physician Assessments [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/residual-symptoms-in-patients-with-psa-who-are-in-very-low-disease-activity-according-to-physician-assessments/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/residual-symptoms-in-patients-with-psa-who-are-in-very-low-disease-activity-according-to-physician-assessments/
