Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: The number of non-inferiority/equivalence randomized clinical trials (NI/EQ-RCTs) in the rheumatoid arthritis (RA) field has recently increased for various reasons. These designs require special considerations and carry the risk of misinterpretation, particularly when they are poorly reported. We conducted a systematic review to assess the reporting quality of RA-related NI/EQ-RCTs over the last decade. We also assessed the reporting quality according to publication period, funding source, and whether the trial was biosimilar-related or not. To the best of our knowledge, this is the first systematic investigation of this kind in the field of rheumatology.
Methods: On May 4, 2024, we searched three databases: (1) Embase, (2) MEDLINE, and (3) the Cochrane Library. Complete published reports of RCTs in RA from 2014 forward that describe the NI/EQ hypothesis involving the primary outcome were included. We excluded phase I/II trials. The reporting quality data form was obtained from key items based on the Consolidated Standards of Reporting Trials statement 2010 extension to NI/EQ-RCTs. We used descriptive statistics to present overall data on reporting quality together with a narrative presentation. To make comparisons, we used the chi-square test and the Fisher exact test, recognizing significance when P < 0.05. This systematic review was prospectively registered in PROSPERO (ID: CRD42024540964) and was not funded by conflicting parties.
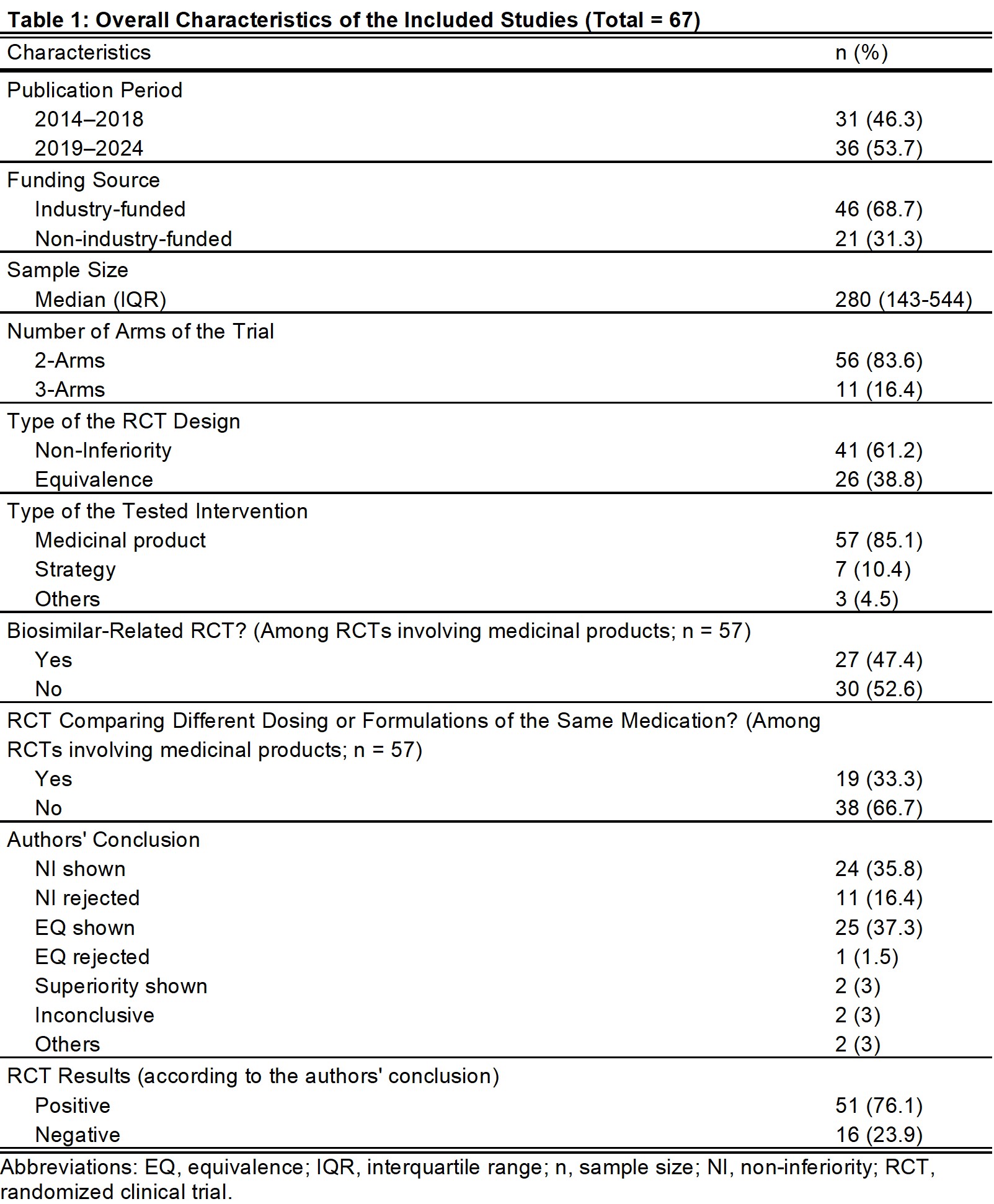
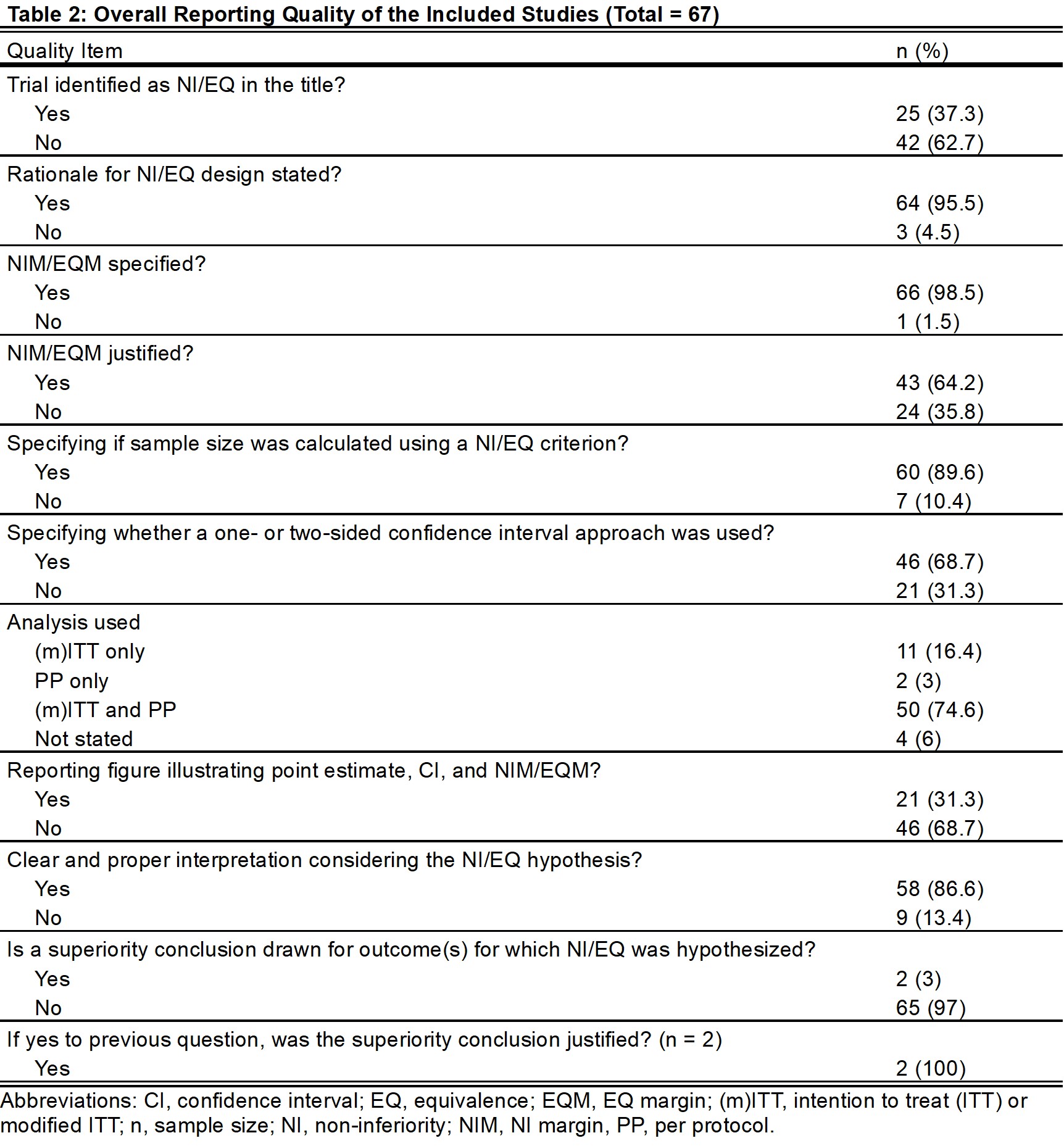
Results: Of 67 studies, 61% and 39% were NI-RCT and EQ-RCT, respectively (Table 1). In total, 25 titles (37%) specified the specific NI/EQ design, 66 studies (99%) specified non-inferiority/equivalence margins (NIM/EQM), and 43 (64%) justified these margins. Fifty studies (75%) reported the results of both intention-to-treat/modified intention-to-treat and per-protocol analyses. Only 21 studies (31%) included the recommended figure illustrating point estimate, confidence interval, and NIM/EQM. Clear interpretations were found in 58 studies (87%; Table 2).
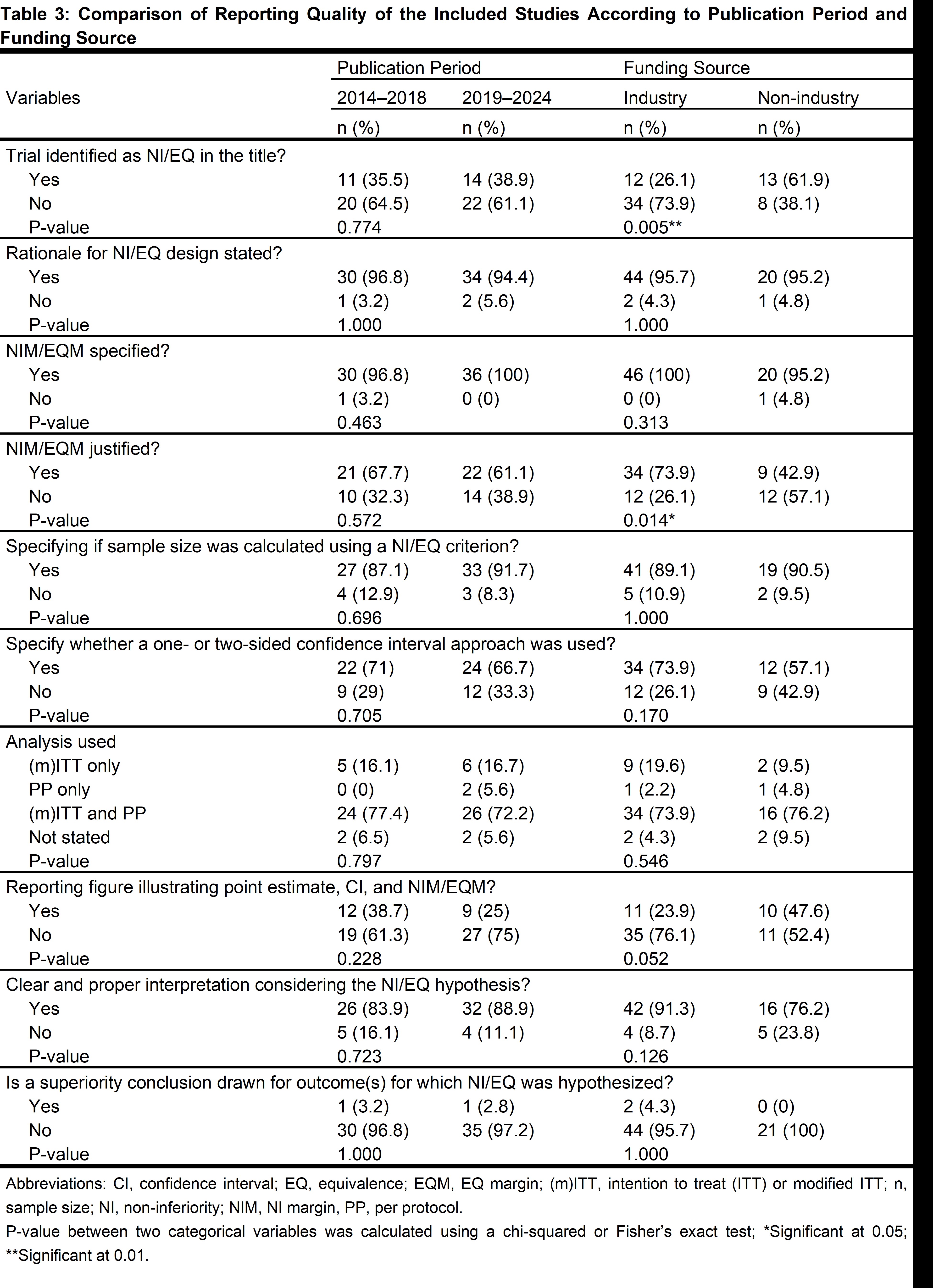
No improvement was found in reporting quality over time between the two halves of this ten-year period (2014-2018 and 2019-2024; Table 3). Industry-funded trials were more likely to justify the NIM/EQM (74% vs 43%; P=0.014) and were less likely to identify the NI/EQ design in the title (26% vs 62%; P=0.005; Table 3). Also, biosimilar-related trials were more likely to justify their NIM/EQM (82% vs 53%; P=0.015) and less likely to identify their design in the title (19% vs 50%; P=0.009).
Conclusion: Overall reporting quality of NI/EQ-RCTs in RA in the last decade is suboptimal, and it is not improving. This is concerning given that these designs are increasingly found in the literature. Clinicians, including rheumatologists, should be aware of the special considerations of these designs and their interpretations. Researchers and journal editors should be more proactive in improving the quality of these reports.
To cite this abstract in AMA style:
Alalwan O, Haider M. Reporting Quality of Non-inferiority and Equivalence Rheumatoid Arthritis Randomized Clinical Trials: A Systematic Review [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/reporting-quality-of-non-inferiority-and-equivalence-rheumatoid-arthritis-randomized-clinical-trials-a-systematic-review/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reporting-quality-of-non-inferiority-and-equivalence-rheumatoid-arthritis-randomized-clinical-trials-a-systematic-review/