Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Systemic Lupus Erythematosus – Treatment I (0801–0806)
Session Type: Abstract Session
Session Time: 1:15PM-1:30PM
Background/Purpose: Cutaneous manifestations of lupus are highly prevalent and have a significant impact on patients’ physical and mental health and wellbeing; however, no targeted therapy is available. Toll-like receptor (TLR) 7/8 signaling has a role in regulating interferon (IFN) induction; elevated type I IFN gene transcripts in lupus skin lesions highlight the importance of the IFN signaling pathway in disease pathogenesis. Enpatoran is a first-in-class oral small molecule TLR 7/8 inhibitor. In a Phase II randomized double-blind placebo-controlled dose-finding parallel adaptive basket study (WILLOW [NCT05162586]), enpatoran improved Cutaneous Lupus Disease Area and Severity Index-Activity (CLASI-A) scores from baseline (BL) and increased CLASI-50 and CLASI-70 response rates in patients with CLASI-A ≥8 SLE and CLE (Cohort A).1 Enpatoran also improved attainment of BICLA response with glucocorticoid taper vs placebo in patients with moderate-to-severe active SLE (Cohort B), despite no statistically significant dose response in this cohort.2 Here we report an exploratory post-hoc analysis of pooled data for all patients from Cohort A and Cohort B who had active cutaneous manifestations (CLASI-A ≥8 at BL).
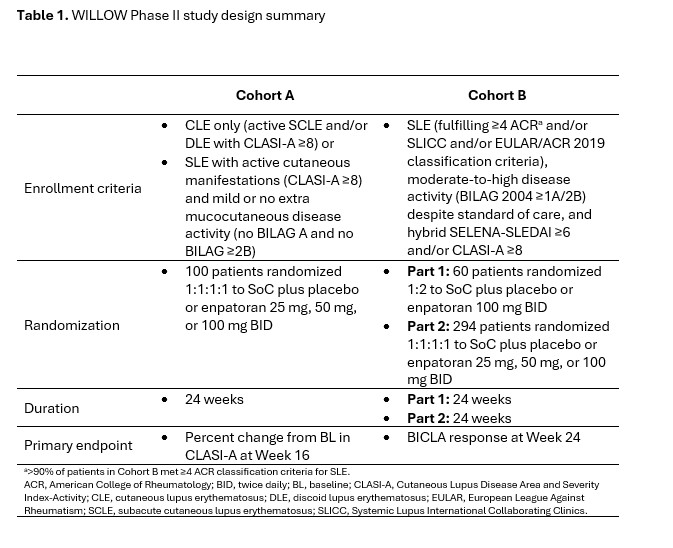
Methods: The WILLOW study design and patient enrollment criteria are summarized in Table 1. Cutaneous outcomes assessed at Week 16 and 24 were predefined secondary/exploratory endpoints in WILLOW and included: CLASI-50 and CLASI-70 response rates, achievement of remission defined as Cutaneous Lupus Activity Investigator’s Global Assessment (CLA-IGA) score 0 or 1, CLASI-A 0–1 or CLASI-A 0–3, and change from BL in Physician Global Assessment (PGA) of cutaneous lupus disease activity.
Results: Baseline demographics and disease characteristics remained balanced across treatment groups in the pooled dataset; 262 patients were analyzed for efficacy in the pooled data (Table 2). Table 3 summarizes the findings for skin outcomes. Of patients receiving enpatoran, up to 71.2% and 78.8% achieved CLASI-50 at Weeks 16 and 24, respectively, vs 37.3% of patients receiving placebo at these timepoints. CLASI-70 responses at Weeks 16 and 24 were achieved by up to 46.3% and 57.1% of patients receiving enpatoran, vs 14.9% and 25.4% who received placebo. A higher proportion of patients in each enpatoran dose group attained remission based on CLA-IGA score 0–1 (nominal p < 0.025 enpatoran 50 mg and 100 mg vs placebo). A higher proportion of patients with remission based on CLASI-A total score with enpatoran was also observed, with up to 30.2%/56.3% achieving CLASI-A 0–1/0–3 (compared with 16.4%/26.9% for placebo) by Week 24. At Week 16 and 24, mean change from BL in PGA of cutaneous lupus disease activity numerically improved across enpatoran doses vs placebo (Table 2).
Conclusion: Patients receiving enpatoran achieved higher rates of CLASI-50/70 response, CLA-IGA and CLASI-A remission vs placebo. Findings support further investigation of enpatoran efficacy and safety in patients with lupus and active cutaneous manifestations.References:1. Pearson DR, et al. Presented at LUPUS 2025. Abstract O010.2. Morand EF, et al. Presented at EULAR 2025. Abstract LB0004.
To cite this abstract in AMA style:
Morand E, Werth V, Furie R, Roy S, Fernandez Ruiz R, Goodson S, Gühring H, Moreau F, Pearson D. Remission from cutaneous manifestations of lupus with enpatoran, a first-in-class oral small molecule toll-like receptor 7/8 inhibitor: pooled post-hoc exploratory analysis from a randomized placebo-controlled Phase II study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/remission-from-cutaneous-manifestations-of-lupus-with-enpatoran-a-first-in-class-oral-small-molecule-toll-like-receptor-7-8-inhibitor-pooled-post-hoc-exploratory-analysis-from-a-randomized-placebo-c/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/remission-from-cutaneous-manifestations-of-lupus-with-enpatoran-a-first-in-class-oral-small-molecule-toll-like-receptor-7-8-inhibitor-pooled-post-hoc-exploratory-analysis-from-a-randomized-placebo-c/

.jpg)
.jpg)