Session Information
Date: Tuesday, November 14, 2023
Title: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes III: Disease Activity
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: A key treatment goal in SLE management is the attainment of remission or LDA,1 for which various definitions exist, including “Definitions of Remission in SLE” (DORIS),2 and “Lupus LDA State” (LLDAS).3Using DORIS and LLDAS criteria, we evaluated remission or LDA attainment in patients with SLE treated with BEL, an approved treatment for active SLE and LN, with standard therapy.
Methods: This is a post hoc analysis of pooled data for adults with SLE from five trials: BLISS-76, BLISS-52, NEA, BLISS-SC, and EMBRACE (GSK Studies BEL110751, BEL110752, BEL113750, BEL112341, and BEL115471). Included patients received BEL (10 mg/kg/month intravenously or 200 mg/week [wk] subcutaneously) or placebo (PBO), plus standard therapy. Attainment of LLDAS and DORIS was compared between BEL and PBO using logistic regression adjusted for trial variance, in the overall population and in the following baseline patient subgroups: SLEDAI 2000 (SLEDAI-2K)score ³10; anti-dsDNA positivity; anti-dsDNA positivity or low complement C3/C4 levels; glucocorticoid (GC) dose (prednisone-equivalent) >7.5 mg/day.
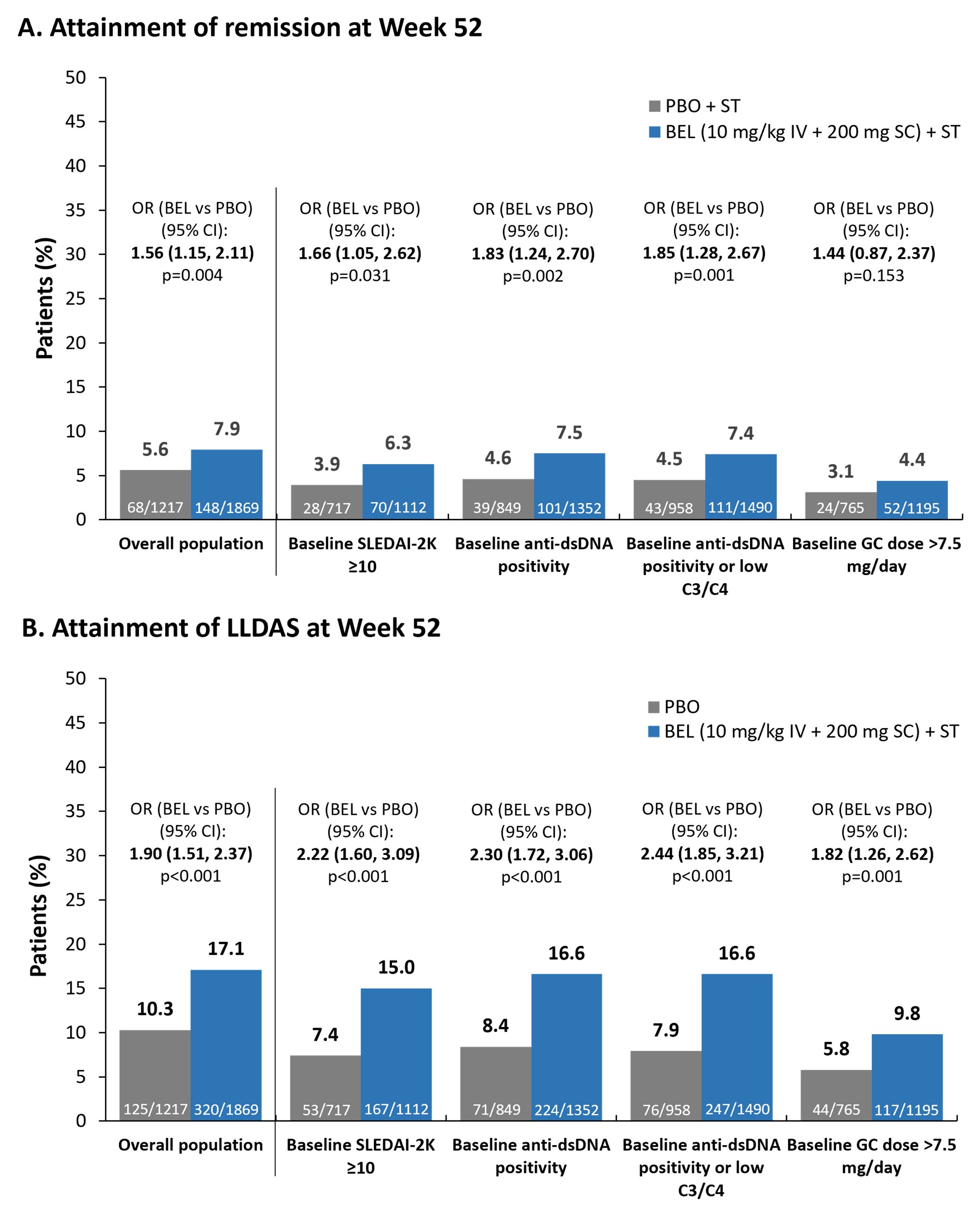
Results: Among 1869 BEL and 1217 PBO patients analyzed, 94.4% were female, mean (standard deviation, SD) age was 37 (12) years. In the overall population, significantly greater attainment of remission was observed for BEL vs PBO at Wk 20 (odds ratio, OR [95% confidence interval, CI]: 1.59 [1.01, 2.50]; p=0.047) and Wk 28 (1.83 [1.20, 2.79]; p=0.005), and BEL superiority was maintained from Wk 48 (1.45 [1.07, 1.95]; p=0.016) to Wk 52 (Figure). Statistically significantly greater attainment of LLDAS with BEL vs PBO was first observed at Wk 24 (1.35 [1.04, 1.74]; p=0.022) and was maintained through Wk 52 (Figure). Similar results were observed for the baseline patient subgroups, with greater remission/LLDAS attainment with longer treatment, except for the GC >7.5 mg/day dose subgroup, where no significant difference in remission attainment was seen with BEL vs PBO (Figure). The earliest superiority of BEL in attaining remission was seen for patients with baselineSLEDAI-2K ³10 at Wk 20 (2.34 [1.11, 4.96]; p=0.026), which was maintained to Wk 52 (Figure). Superiority of BEL in attaining LLDAS was first seen at Wk 16 for patients with baselineSLEDAI-2K ³10 (1.65 [1.01, 2.69]; p=0.045), baseline anti-dsDNA positivity (1.48 [1.01, 2.18]; p=0.043), and baseline anti-dsDNA positivity/low C3/C4 (1.49 [1.04, 2.12]; p=0.029), and was maintained from Wk 24 (1.78 [1.20, 2.65]; p=0.004, 1.46 [1.05, 2.02]; p=0.025, and 1.49 [1.09, 2.02]; p=0.011, respectively) to Wk 52 (Figure).
Conclusion: In this large analysis, statistically significant differences in attainment of remission and LLDAS in favor of BEL vs PBO were seen as early as 24 wks after treatment, with 8% (vs 6%) and 17% (vs 10%) of BEL-treated patients achieving remission and LLDAS, respectively, at Wk 52. BEL appeared particularly beneficial for patients with baselineSLEDAI-2K ³10 and baseline anti-dsDNA positivity/low C3/C4, in whom LLDAS was attained earlier than in the overall population.
Funding: GSK
References
1 Fanouriakis A et al. Ann Rheum Dis 2019;78:736–45
2 van Vollenhoven RF et al. Lupus Sci Med 2021;8:e000538
3 Franklyn K et al. Ann Rheum Dis 2016;75:1615–21
*The DORIS remission definition requires a clinical SLEDAI_2K score=0 and a PGA score <0.5 (scale 0–3), while it allows serological activity and use of low-dose GCs (prednisone-equivalent <=5 mg/day) and immunosuppressive or biological agents at standard doses; †the LLDAS definition requires a SLEDAI_2K score <=4, excluding major organ activity and fever or new activity since the previous assessment, and PGA score <=1 (scale 0–3), and it allows a prednisone-equivalent dose <=7.5 mg/day and immunosuppressive or approved biological agents at standard doses.
IV, intravenous; PGA, Physician Global Assessment; SC, subcutaneous; ST, standard therapy.
To cite this abstract in AMA style:
Parodis I, Lindblom J, Levy R, Zen M, Cetrez N, Gomez A, Oon S, Henning C, Khamashta M, Quasny H, Chauhan D, Askanase A, van Vollenhoven R, Nikpour M. Remission and Low Disease Activity (LDA) in Patients with SLE Treated with Belimumab (BEL): Results from a Large Integrated Analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/remission-and-low-disease-activity-lda-in-patients-with-sle-treated-with-belimumab-bel-results-from-a-large-integrated-analysis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/remission-and-low-disease-activity-lda-in-patients-with-sle-treated-with-belimumab-bel-results-from-a-large-integrated-analysis/