Session Information
Date: Sunday, November 5, 2017
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Vaccine-preventable infections, including reactivation of hepatitis B virus, are a leading cause of morbidity and mortality in immunocompromised patients. Guidelines recommend that all immunosuppressed patients should be screened with hepatitis B serologies for active or chronic infection as well as need for repeat vaccination. Previously at our center, we have implemented hepatitis B serology screenings for patients on intravenous biologics and pneumococcal vaccines for all immunocompromised patients. The aim of this intervention was to adapt these processes to hepatitis B serology screenings and vaccinations for all immunocompromised pediatric rheumatology patients.
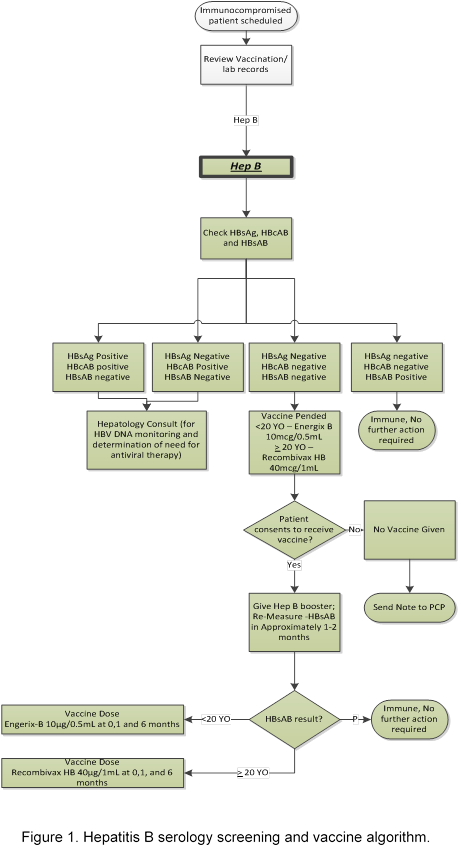
Methods: The Model for Improvement was used to form a team, map the process, construct a key driver diagram, and develop an algorithm (Figure 1). Eligible patients included those over 7 years identified as immunocompromised using a validated algorithm in the electronic health record (EHR). A series of Plan-Do-Study-Act (PDSA) cycles were performed to test and implement the multi-step process. After building an electronic order-set in the EHR, we adopted the process of clinic staff pending needed orders during pre-visit planning. We also provided education and developed “talking points” for clinic staff and providers. Results were tracked on a statistical process control chart weekly, and failures were identified through Pareto analysis.
Results: The intervention began in December 2016 at a tertiary care pediatric rheumatology clinic. Prior to start, a subset of patients had previously received screenings and vaccines due to an initial process for patients on intravenous biologics. However, by systematically adopting interventions, we were able to rapidly and reliably achieve our goal performance (Figure 2). There were few failures for the serology screenings from orders not signed. Failures for vaccine administration included deferred, primary care preference, refusal, leaving before vaccine, and vaccine orders not signed. To date, we have screened 862 patients and administered 302 booster vaccines.
Conclusion: By adapting processes that were previously successful in our clinic setting, we were able to reliably implement a hepatitis B serology screening and vaccination program over a short time-frame. Next steps include sustainability planning and spread to other clinics with immunocompromised patients. The results of this project support the importance of adoption and spread of successful processes in order to expedite improvement in quality of care.
To cite this abstract in AMA style:
Smitherman EA, Furnier A, Watts A, Kramer S, Baker EJ, Dykes DM, Brady R, Huggins JL. Reliable Implementation of a Hepatitis B Serology Screening and Vaccination Process for Immunocompromised Pediatric Rheumatology Patients [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/reliable-implementation-of-a-hepatitis-b-serology-screening-and-vaccination-process-for-immunocompromised-pediatric-rheumatology-patients/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reliable-implementation-of-a-hepatitis-b-serology-screening-and-vaccination-process-for-immunocompromised-pediatric-rheumatology-patients/