Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The Self‐Administered Comorbidity Questionnaire (SCQ) is a self-report questionnaire. It assesses 13 common medical conditions and the impact of comorbidities on functional status (1). The aim of this study is to investigate the validity and reliability of the Turkish version of the SCQ in Psoriatic Arthritis (PsA).
Methods: Patients with PsA, according to Classification Criteria for Psoriatic Arthritis (CASPAR), were included in the study. Data about age, sex, body mass index (BMI), disease duration (month) were noted. Psoriatic Arthritis Quality of Life (PsAQoL) scale and Short Form 36 (SF-36) were used to assess the quality of life. The Health Assessment Questionnaire (HAQ) was used to evaluate the physical disability. The effects of comorbid diseases on quality of life (QoL) and Patient Global Assessment of General Health (PGA) were assessed on the Visual Analogue Scale (VAS). The English version of SCQ was the original scale used for translation and adaptation. Translation, back translation, and cross-cultural adaptation of the SCQ into Turkish were done according to the standard procedure. The reliability of SCQ was determined by internal consistency (Cronbach’s alpha coefficient). Face validity and construct validity (convergent and divergent validities) were evaluated. For the face validity, the final Turkish version of SCQ was tested by 15 patients to determine if they could understand the items. The correlations of the SCQ with the HAQ, SF-36, PsAQoL, the VAS scores of the effect of comorbid diseases on QoL and PGA were assessed for convergent validity. The relation of the SCQ with age, BMI, and disease duration were assessed for divergent validity. The construct validity of the SCQ scale was determined by Spearman’s correlation coefficient. The descriptive analysis was done for demographic data. P< 0.05 accepted as significant.
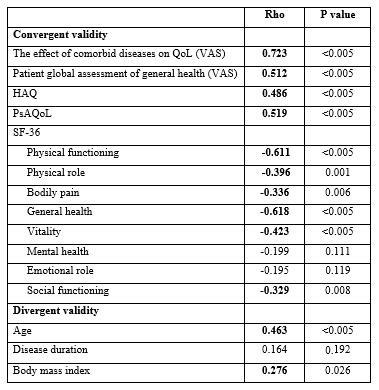
Results: The mean age of 65 patients (46 female, 19 male) with PsA was 46.20 (SD: 13.05) years. The median (min-max) duration of disease was 24 (1-384) months. The mean BMI of patients was 29.06 (SD: 5.92). The Cronbach’s alpha coefficient of the SCQ for internal consistency was 0.755. The SCQ score had moderate and significant positive correlations with HAQ, PsAQoL, the VAS scores of the effect of comorbidity on QoL and PGA (p< 0.05). The significant negative correlations were detected with physical function, physical role limitation, bodily pain, general health, vitality and social function subgroups of the SF-36 (p< 0.05). The SCQ score had positive correlations with age and BMI (p< 0.05). There was no correlation between SCQ score and duration of disease (rho: 0.164, p: 192). The correlations of SCQ with the clinical variables that show convergent and divergent validities were given in Table 1.
Conclusion: The Turkish version of the SCQ is a valid and reliable instrument in PsA. The SCQ is a simple, accurate and not time-consuming self-report instrument to assess comorbidities in patients with PsA.
Reference:
1. Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003; 49(2):156-63.
To cite this abstract in AMA style:
Erdem D, Gezer H, Acer Kasman S, Duruoz M. Reliability and Validity of the Self‐Administered Comorbidity Questionnaire in Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/reliability-and-validity-of-the-self%e2%80%90administered-comorbidity-questionnaire-in-psoriatic-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reliability-and-validity-of-the-self%e2%80%90administered-comorbidity-questionnaire-in-psoriatic-arthritis/