Session Information
Date: Tuesday, November 10, 2015
Title: Spondylarthropathies and Psoriatic Arthritis - Clinical Aspects and Treatment Poster III: Therapy
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The phase 3 FUTURE 1 and FUTURE
2 trials demonstrated efficacy of secukinumab (SEC) for active psoriatic
arthritis (PsA). However, there is limited data comparing the effectiveness of
SEC with established PsA treatments. This study compared the relative efficacy of adalimumab (ADA) 40mg versus SEC
150mg and 300mg for the treatment of PsA.
Methods: An indirect comparison was
conducted using individual patient data from the ADA pivotal trial (ADEPT) and
published data from FUTURE 1 and 2. To adjust for the cross-trial differences, individual
patients in ADEPT were re-weighted to match the mean baseline characteristics in
the FUTURE trials.1,2 Specifically, age, weight, gender, race, baseline
methotrexate use, psoriasis ≥ 3% body surface area, baseline PASI score,
presence of dactylitis and enthesitis, and HAQ-DI were included in the baseline
matching. The ACR 20/50/70 and PASI 75/90 response rates relative to placebo at
week 24 were compared. Numbers needed to treat (NNTs) to achieve one additional
ACR 20 and PASI 75 responder were also calculated.
Results: After matching, mean baseline
characteristics were balanced across the ADEPT and FUTURE 1 and 2 trial
populations. For all outcomes, ADA demonstrated a higher relative efficacy to
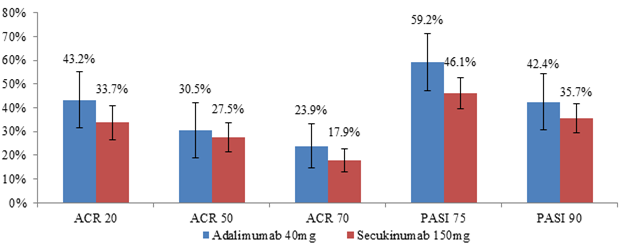
placebo when compared to SEC 150mg (n=302) (Figure 1). Specifically, the mean
differences (95% confidence interval) between ADA and SEC 150mg in relative ACR
20/50/70 response rates were 9.5% (-4.3%, 23.3%), 3.0% (-10.1%, 16.0%), and
6.0% (-4.4%, 16.5%), respectively. The mean differences in relative PASI 75/90
response rates were 13.1% (-0.5%, 26.7%) and 6.7% (-6.4%, 19.9%). Similarly,
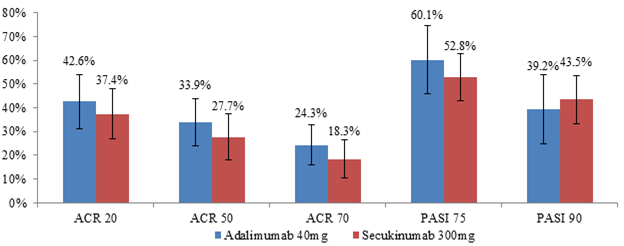
ACR 20/50/70 and PASI 75 were higher with ADA vs SEC 300mg (n=100) (Figure 2).
The NNTs to achieve one additional ACR 20 responder were 2.3 for ADA vs 3.0 for
SEC 150mg and 2.7 for SEC 300mg. The NNTs to achieve one additional PASI75
responder were also lower for ADA (1.7) vs SEC 150mg (2.2) and SEC 300mg (1.9).
Conclusion: In the absence of head to head
trials comparing ADA and SEC,
the current indirect comparison which adjusts for differences across trial
populations provides
valuable and reliable evidence for physicians and payers. After adjusting for
cross-trial differences in baseline characteristics, ADA was associated with higher
relative ACR and PASI rates and lower NNTs compared with SEC 150mg or 300mg at
week 24 among patients with PsA.
1 Signorovitch
et al. Pharmacoeconomics 2010; 28 (10): 935-945.
2 Signorovitch et
al. Value in Health 2012; 15 (6), 940-947.
Figure 1
ACR 20/50/70 and PASI 75/90 relative to placebo of ADA 40mg vs. SEC 150mg
Figure 2
ACR 20/50/70 and PASI 75/90 relative to placebo of ADA 40mg vs. SEC 300mg
To cite this abstract in AMA style:
Betts KA, Mittal M, Joshi A, Song J, Bao Y. Relative Efficacy of Adalimumab Versus Secukinumab in Active Psoriatic Arthritis: A Matching-Adjusted Indirect Comparison [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/relative-efficacy-of-adalimumab-versus-secukinumab-in-active-psoriatic-arthritis-a-matching-adjusted-indirect-comparison/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/relative-efficacy-of-adalimumab-versus-secukinumab-in-active-psoriatic-arthritis-a-matching-adjusted-indirect-comparison/