Session Information
Date: Sunday, October 21, 2018
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Psoriatic arthritis (PsA) is a heterogeneous inflammatory condition characterized by the clinical domains of arthritis, enthesitis, dactylitis, spondylitis, and psoriasis. While it is known that both psoriasis and articular/periarticular symptoms have a meaningful impact on lives of PsA patients, it is not known the degree to which improvements in these symptoms independently contribute to improvements in quality of life (QoL) or function. This analysis quantifies the contributions of improvements in joint related symptoms (Disease Activity Index for PsA [DAPSA]) and skin related symptoms (Psoriasis Area and Severity Index [PASI]) to improvements in function (Health Assessment Questionnaire and Disability Index [HAQ-DI]) and skin-related quality of life (Dermatology Life Quality Index [DLQI]) using a multi-mediator analysis.
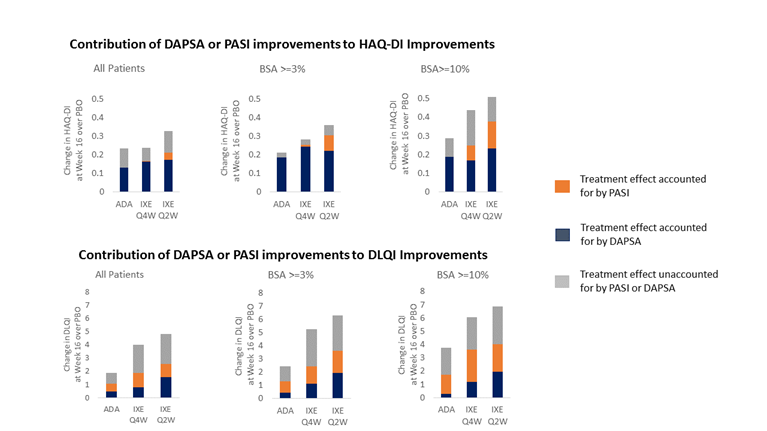
Methods: This analysis included data from a Phase 3 randomized clinical trial (SPIRIT-P1; NCT01695239) in biologic DMARD-naïve patients who met criteria for PsA who were randomly assigned to treatment groups (ixekizumab 80 mg every 4 weeks [IXEQ4W, N=107], every 2 weeks [IXEQ2W, N=103], adalimumab [ADA, N=101] or placebo [PBO, N=106]). In this analysis, a multiple mediation model1 was employed to assess the ‘direct’ and ‘indirect (mediation) effects of treatment on the DLQI and HAQ-DI at week 16 using percent improvement in DAPSA and PASI as the mediators. In the model, the indirect effects represent how much of the improvements in HAQ-DI or DLQI can be attributed to improvements in PASI or DAPSA. The direct effects represent the mean improvement in HAQ-DI or DLQI that cannot be accounted for by DAPSA or PASI improvements. Analyses were conducted on the intent-to-treat population and in patients with ≥3% and ≥10% body surface area (BSA) psoriasis involvement. Missing data was imputed using the last observation carried forward.
Results: The relative contributions of DAPSA or PASI improvements to improvements in HAQ-DI and DLQI for each treatment arm over placebo are shown in the Figure. Improvements in DAPSA accounted for the majority of improvements observed in HAQ-DI, and improvements in PASI accounted for the majority of improvements in DLQI. In patients with moderate to severe (BSA≥3%) or severe (BSA≥10%) psoriasis, PASI had higher levels of contribution to both HAQ-DI and DLQI.
Conclusion: These results indicate that improvements in both joint and skin symptoms play a role in improving different aspects of function and QoL for PsA patients. While DAPSA was the most substantial contributor to improvements in HAQ-DI, the skin also plays a role, potentially through the effect of skin symptoms such as itching and skin pain on some domains of the HAQ-DI such as grooming or hygiene.
1Preacher KJ, Hayes AF. Behavior Research Methods. 2008; 40(3):879-891.
To cite this abstract in AMA style:
Ogdie A, Sunkureddi P, Zhu B, Spraberry A, Malatestinic W, Dong Y, Shrom D. Relative Contributions of Improvements in the Psoriasis Area and Severity Index (PASI) and Disease Activity Index for Psoriatic Arthritis (DAPSA) to Improvements in Quality of Life and Function in Patients with Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/relative-contributions-of-improvements-in-the-psoriasis-area-and-severity-index-pasi-and-disease-activity-index-for-psoriatic-arthritis-dapsa-to-improvements-in-quality-of-life-and/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/relative-contributions-of-improvements-in-the-psoriasis-area-and-severity-index-pasi-and-disease-activity-index-for-psoriatic-arthritis-dapsa-to-improvements-in-quality-of-life-and/