Session Information
Date: Tuesday, November 9, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster III: Psoriatic Arthritis II (1801–1835)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Fatigue is a key patient (pt)-reported symptom of psoriatic arthritis (PsA).1,2 Utilizing data from the Phase 3 DISCOVER-1 (D1) and -2 (D2) studies, these post hoc analyses explored: 1) correlation of fatigue with systemic inflammation (CRP) and hemoglobin (Hgb); 2) relationship between improvements in fatigue and clinical outcomes with guselkumab (GUS), a selective IL-23p19 inhibitor.
Methods: Pts with active PsA despite standard therapies in D1 (swollen joint count [SJC] ≥3, tender joint count [TJC] ≥3, CRP ≥0.3 mg/dL) and D2 (SJC ≥5, TJC ≥5, CRP ≥0.6 mg/dL) were randomized 1:1:1 to GUS 100 mg Q4W; GUS 100 mg at W0, W4, then Q8W; or placebo (PBO) with crossover to GUS 100 mg Q4W at W24 (PBO͢àQ4W). Fatigue was evaluated using the Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-F) scale (0–52 [higher score=less fatigue]; clinically meaningful improvement ≥4 points). FACIT-F scores, as well as their correlation (Pearson) with CRP and Hgb were determined in pts with anemia (Hgb < 13.5 [males] or < 12 [females] g/dL) and without anemia through W24. Relationships between FACIT-F score (outcome) and CRP/Hgb (predictors, along with visit) were assessed via a mixed model for repeated measures (MMRM). Associations between FACIT-F response in GUS-treated pts (Q4W+Q8W+PBOàQ4W) and achievement of American College of Rheumatology (ACR) 20/50/70, Health Assessment Questionnaire Disability Index (HAQ-DI), and Minimal Disease Activity (MDA) responses were evaluated at W52 for D1/D2 and at W100 for D2 (nonresponder imputation).
Results: In pooled analyses (N=1120), mean baseline (BL) FACIT-F scores for 583 males (31.3) and 537 females (28.5) were
Conclusion: In pts with active PsA, systemic inflammation and Hgb levels were key predictors of FACIT-F scores. FACIT-F responders were more likely to achieve favorable clinical outcomes through up to 2 years of GUS.
References
1. Leung YY, et al. J Rheumatol (Suppl) 2020;96:46-9.
2. Gudu T, et al. Joint Bone Spine 2016;83:439-43.
3. Montan I, et al. Value Health 2018;21:1313-21.
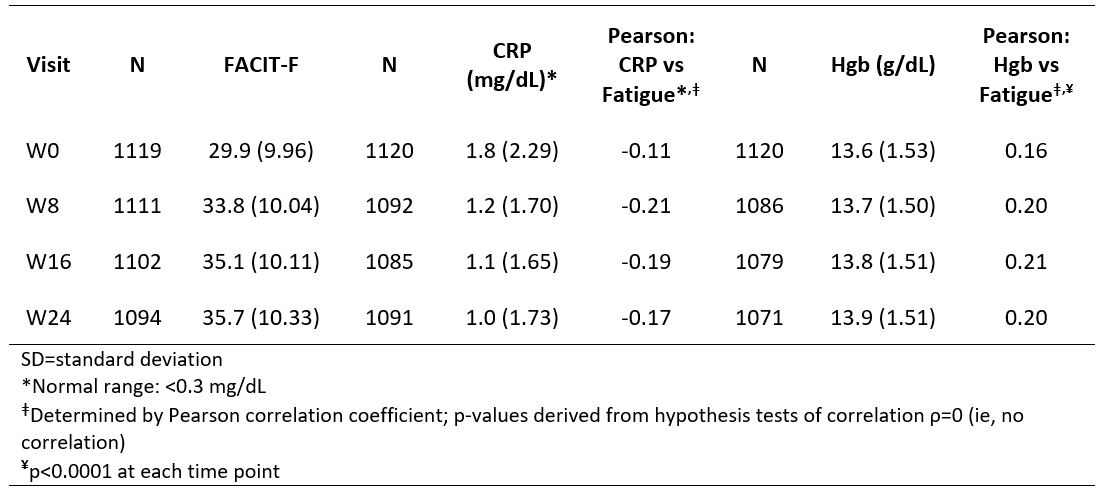 Table. Mean (SD) of FACIT-F Scores and CRP/Hgb Levels by Visit: Pooled DISCOVER_1 and _2 Studies
Table. Mean (SD) of FACIT-F Scores and CRP/Hgb Levels by Visit: Pooled DISCOVER_1 and _2 Studies
 Figure. Achievement of ACR20/50/70, HAQ-DI, and MDA Responses by FACIT-F Response Status Among GUS-treated Patients at W52 and W100 of DISCOVER_2
Figure. Achievement of ACR20/50/70, HAQ-DI, and MDA Responses by FACIT-F Response Status Among GUS-treated Patients at W52 and W100 of DISCOVER_2
To cite this abstract in AMA style:
Rahman P, Mease P, Deodhar A, Gossec L, Kavanaugh A, Chakravarty S, Kollmeier A, Liu Y, Lin X, Shawi M, Han C. Relationships Between Fatigue and Hemoglobin/C-Reactive Protein Levels and Associations Between Fatigue and Clinical Response in Patients with Active Psoriatic Arthritis: Results from Two Randomized Controlled Trials of Guselkumab (TREMFYA®) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/relationships-between-fatigue-and-hemoglobin-c-reactive-protein-levels-and-associations-between-fatigue-and-clinical-response-in-patients-with-active-psoriatic-arthritis-results-from-two-randomized-c/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/relationships-between-fatigue-and-hemoglobin-c-reactive-protein-levels-and-associations-between-fatigue-and-clinical-response-in-patients-with-active-psoriatic-arthritis-results-from-two-randomized-c/
