Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Uncontrolled RA activity and acute disease flares are associated with higher risk of adverse outcomes, such as cardiovascular (CV) disease, venous thromboembolism (VTE), malignancy, and infection.1-4 Here, we evaluate associations of acute and cumulative Clinical Disease Activity Index (CDAI) measurements with CV, malignancy, or infectious adverse events (AEs) of interest in ORAL Surveillance.
Methods: ORAL Surveillance (NCT02092467) was a post-authorization safety study of tofacitinib vs TNF inhibitors (TNFi) in patients aged ≥ 50 years with active RA despite MTX and ≥ 1 additional CV risk factor. Patients were randomized 1:1:1 to receive tofacitinib 5 or 10 mg twice daily (BID) or subcutaneous TNFi. Two post hoc analyses were performed: (1) a time-varying multi-variable Cox model examining risks of AEs of interest when patients were in CDAI-defined low ( > 2.8–≤ 10; LDA), moderate ( > 10–≤ 22; MDA), or high ( > 22; HDA) disease activity vs remission ( ≤ 2.8), and including patient demographics, medical history, RA characteristics, prior treatments, baseline medications, and treatment arm, pre-selected using backward selection, and (2) calculation of area under the curve (AUC)/year for CDAI prior to event or to study end (patients without events) and comparison using analysis of variance with treatment arm, event status, and interaction between the two (supportive). Nominal p values were generated.
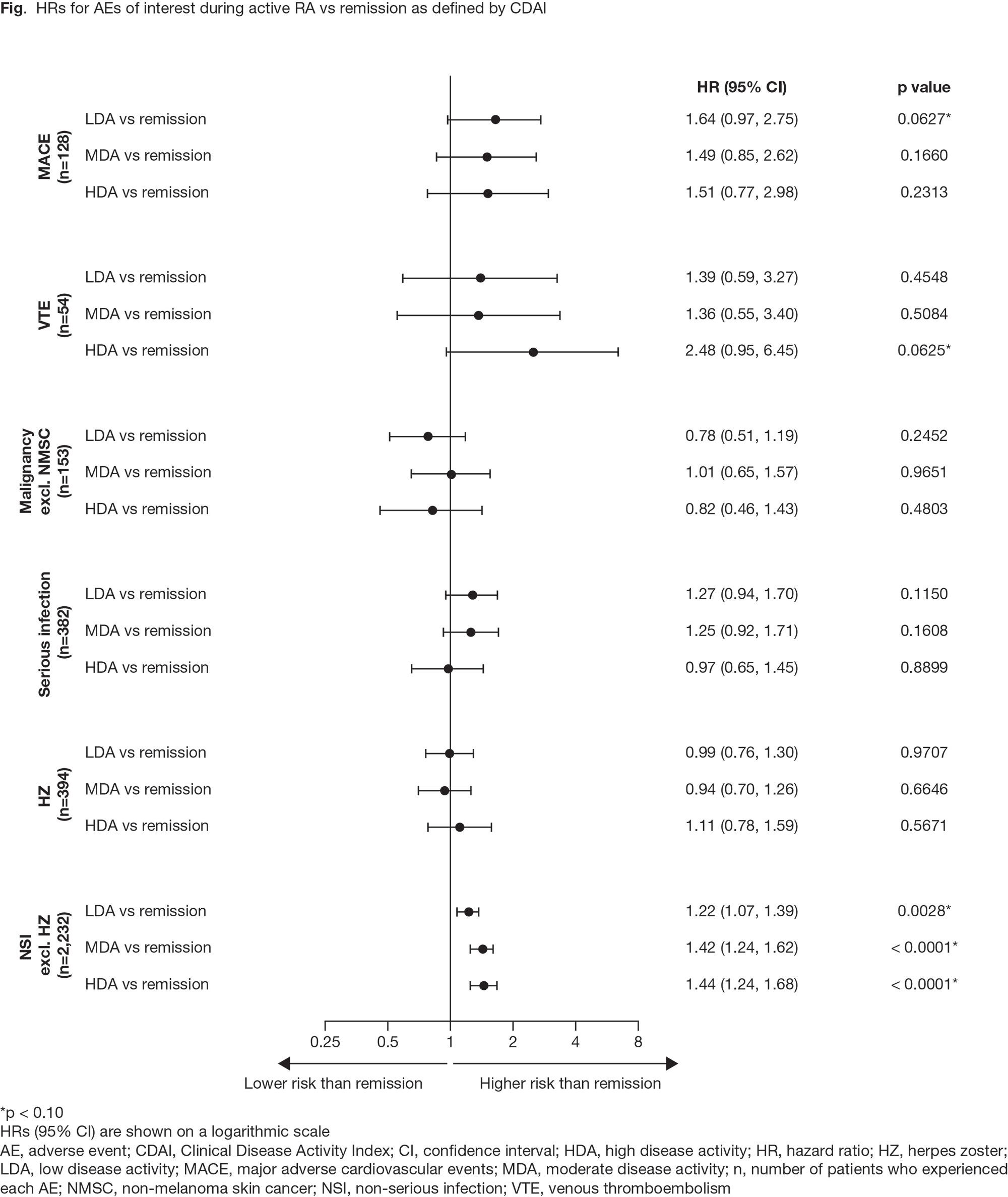
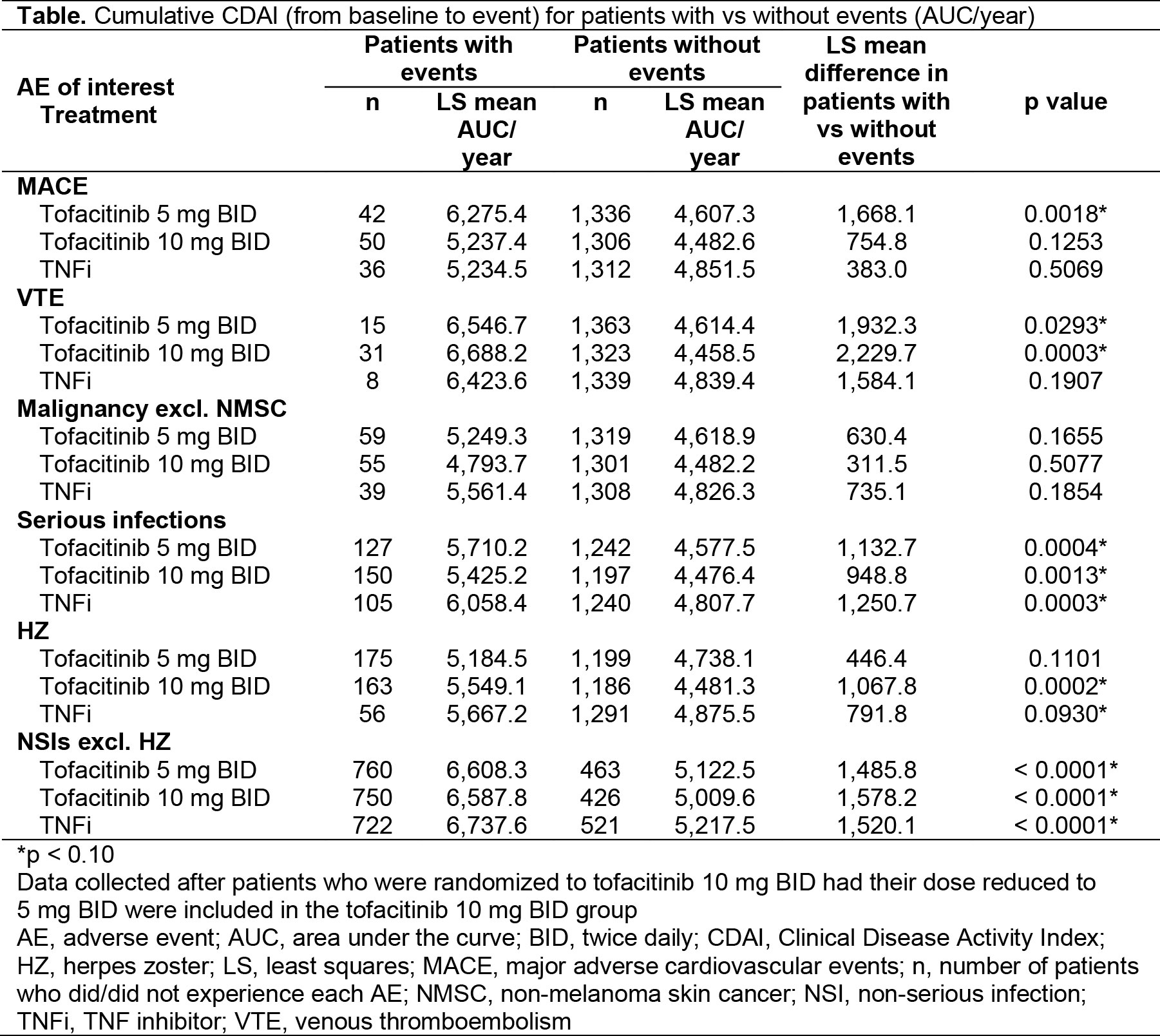
Results: A total of 4,362 patients were included. Mean RA duration at baseline was approximately 10 years. All patients were on MTX at baseline, and 28% had previously been on one other synthetic DMARD. Overall, 10% of patients had been on one biologic DMARD. Hazard ratios suggested that when patients had LDA, MDA, or HDA vs remission, they were potentially at higher risk of developing major adverse CV events (MACE), VTE, and non‑serious infections (NSIs; excluding herpes zoster [HZ]), but not malignancies, serious infections, or HZ (Fig). Similarly, mean CDAI AUC trended higher for MACE, VTE, and NSIs for patients with events (Table).
Conclusion: In ORAL Surveillance, the risk of MACE, VTE, and NSIs excluding HZ, was higher when patients had active disease vs remission. Greater cumulative RA disease activity was seen in patients with these AEs vs those without. Our findings support treat‑to-target recommendations for RA.
1. Molander et al. Ann Rheum Dis 2021; 80: 169-75
2. Maradit-Kremers et al. Arthritis Rheum 2005; 52: 722-32
3. Au et al. Ann Rheum Dis 2011; 70: 785-91
4. Baecklund et al. Arthritis Rheum 2006; 54: 692-701
Study sponsored by Pfizer. Medical writing support was provided by K Thompson, CMC Connect, and funded by Pfizer.
To cite this abstract in AMA style:
Karpouzas G, Szekanecz Z, Baecklund E, Mikuls T, Bhatt D, Shi H, Wang C, Sawyerr G, Chen Y, Menon S, Connell C, Ytterberg S, Mortezavi M. Relationship Between Disease Activity and Adverse Events of Interest in Patients with RA on Tofacitinib or TNF Inhibitors: Post Hoc Analysis of a Phase 3b/4 Randomized Safety Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/relationship-between-disease-activity-and-adverse-events-of-interest-in-patients-with-ra-on-tofacitinib-or-tnf-inhibitors-post-hoc-analysis-of-a-phase-3b-4-randomized-safety-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/relationship-between-disease-activity-and-adverse-events-of-interest-in-patients-with-ra-on-tofacitinib-or-tnf-inhibitors-post-hoc-analysis-of-a-phase-3b-4-randomized-safety-study/