Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Biologic treatments such as monoclonal antibodies have become first line treatment for many autoimmune diseases and now monoclonal antibodies are being used to treated hyperlipidemia, osteoporosis etc. There is a paucity of evidence in understanding combination biologic use in a single patient. Historically, combination biologic treatments have demonstrated harm primarily due to an increased risk of infection. New research suggests that patients might tolerate combination immunosuppressive and non-immunosuppressive biologics for treatment of different conditions. This study will use claims data to evaluate combination immunosuppressive and non-immunosuppressive biologics and incidence of emergency room visits.
Methods: This is a U.S. multicenter retrospective chart review study conducted using MarketScan database. The data was used to evaluate patients starting a single biologic treatment and patients starting combination immunosuppressant and non-immunosuppressant biologic treatments, and comparing the incidence of emergency room visits within a 180 day period. Chi-squared analysis was used.
Inclusion criteria: adults, age >65 years, who have received single biologic or combination immunosuppressive and non-mmunosuppressive biologics from 1/1/2012 to 12/31/2020. Certain biologics were categorized unconventionally.
Immunosuppressive biologics were identified by their unique J-codes and included: Adalimumab, basiliximab, belimumab, canakinumab, certolizumab, daclizumab, etanercept, golimumab, guselkumab, infliximab, natalizumab, ocrelizumab, rituximab, siltuximab, tildrakizumab, tocilizumab, ustekinumab, andvedolizumab.
Non-immunosuppressives with unique J-codes included: Abciximab, benralizumab, bevacizumab, bezlotoxumab burosumab‐Twza, denosumab, omalizumab, mepolizumab, reslizumab, romosozumab, and teprotumumab‐Trbw.
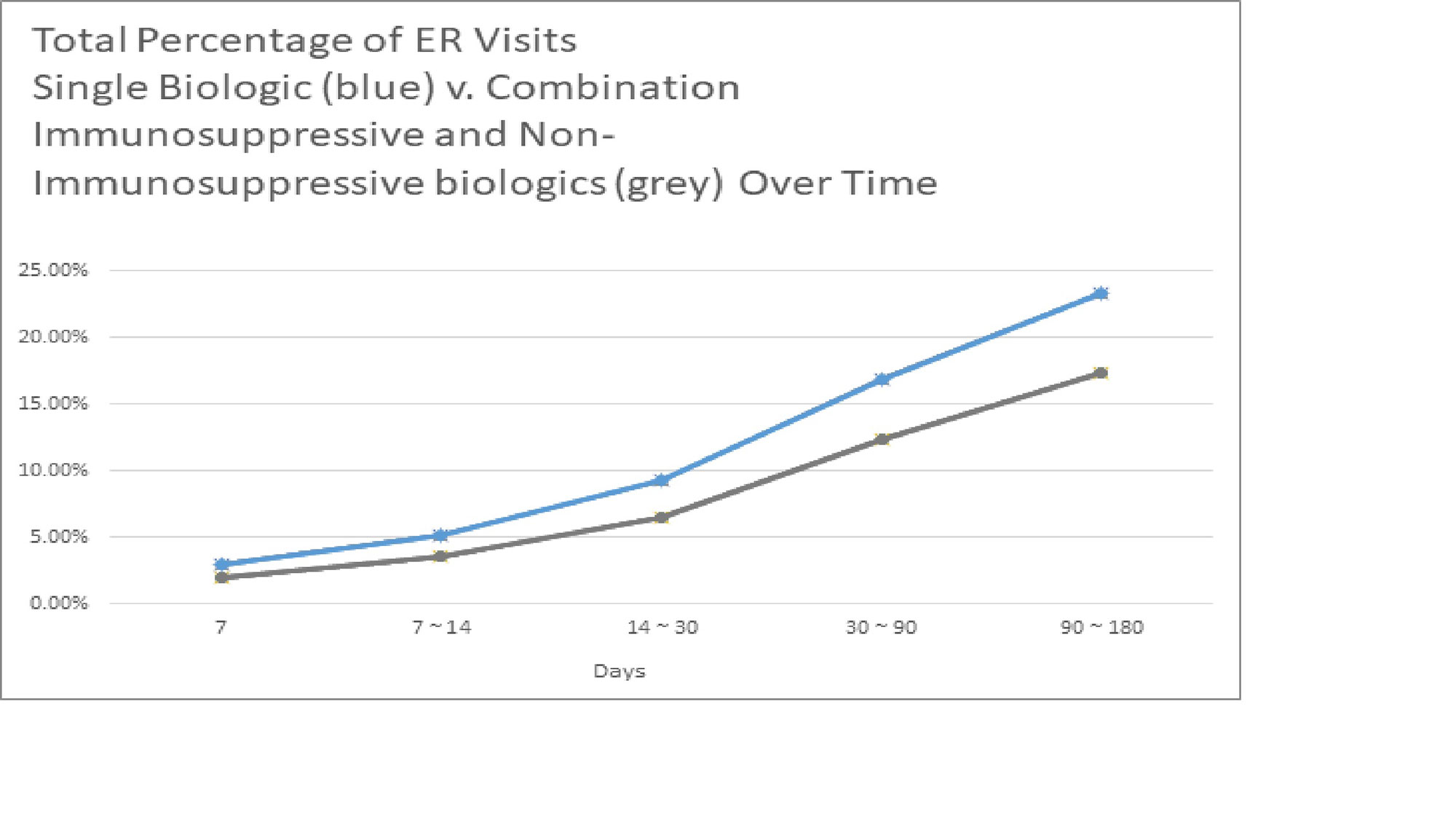
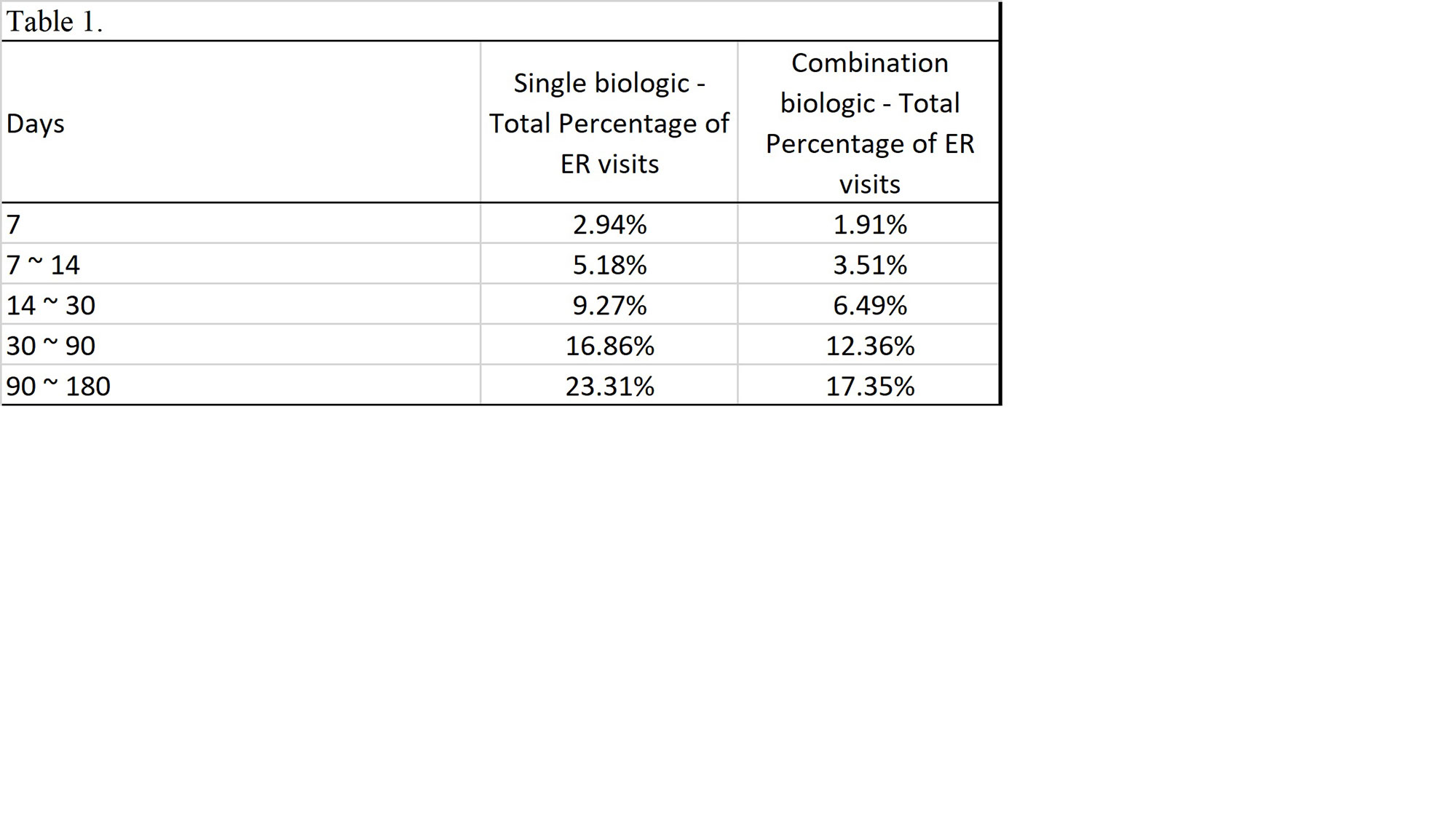
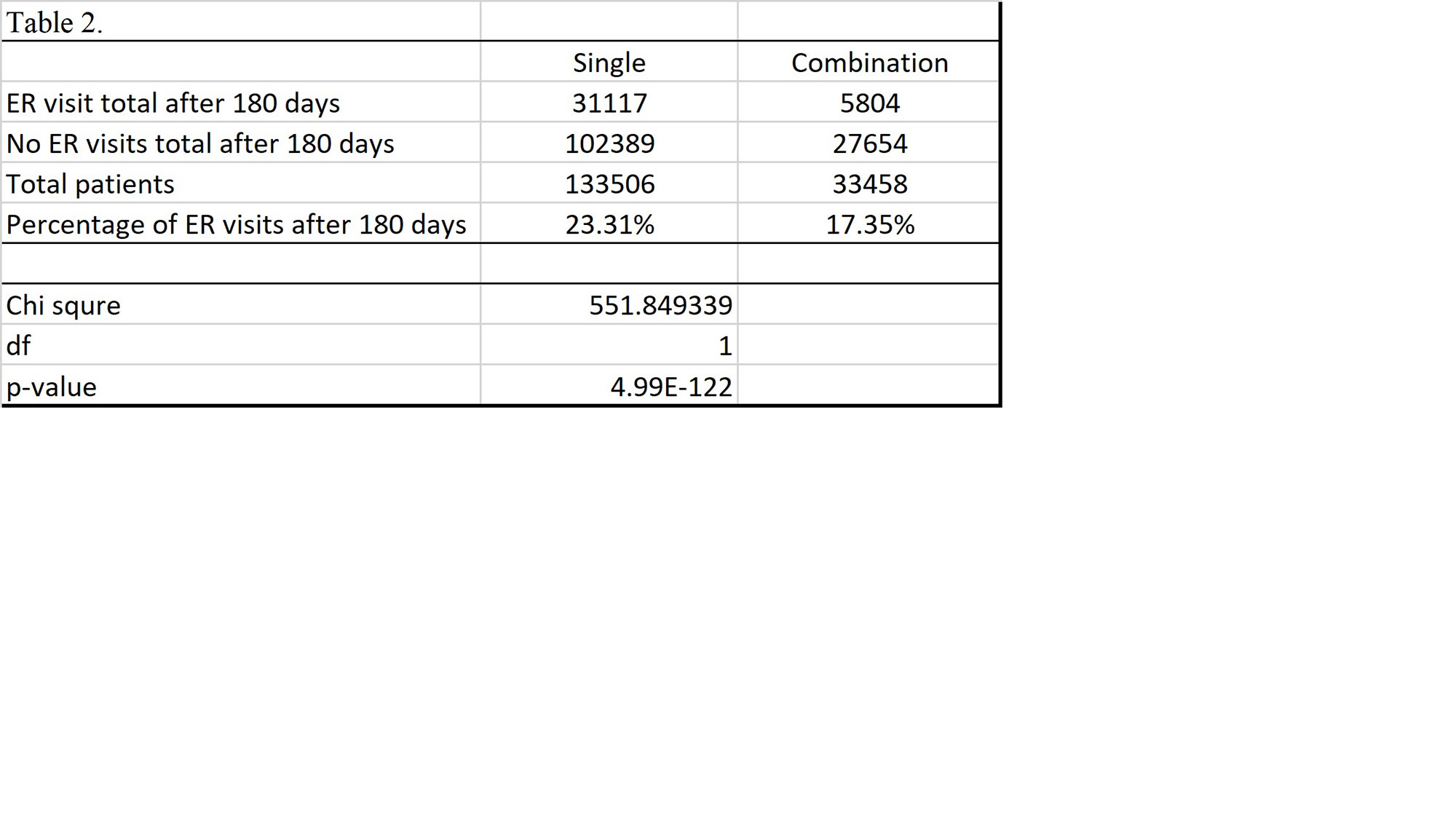
Results: Total of 133,506 patients were identified that had received a single biologic. Of those receiving a single biologic agent only 31,117 patients or 23.31% had an ER visit within 180 days. Total 33,458 patients receiving combination biologics were identified. The total ER visits within 180 days of those receiving combination biologics was 5,804 patients or 17.35%. The p-value of chi-square test is < 0.01 comparing ER visits or no ER visits between the single biologic v. combination biologic group.
Conclusion: This large multicenter data demonstrates that in regards to emergency room visits, there is a statistically significant difference between patients on single biologic and combination immunosuppressive and non-immunosuppressive biologic treatments; unexpectedly, the incidence of ER visits within a 180 day period was lower in patients receiving combination biologics than single biologics. Further inquiry is required to determine if certain combinations biologics have a safer risk profile than others. Many biologics were not included in study due lack of unique J-code. Moreover, additional research is required to determine if there are any other factors that contributed to the disparity of ER visits.
To cite this abstract in AMA style:
Mathew R, Meara A, Chiang C, Paradakar K, Burrell A, Kirsch G. Reevaluating Clinical Outcomes of Patients on Combination Biologics [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/reevaluating-clinical-outcomes-of-patients-on-combination-biologics/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reevaluating-clinical-outcomes-of-patients-on-combination-biologics/