Session Information
Date: Sunday, November 8, 2020
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: In the randomized placebo-controlled INBUILD trial in patients with chronic fibrosing ILDs with a progressive phenotype, nintedanib reduced the rate of decline in forced vital capacity (FVC) over 52 weeks, with an adverse event profile characterized mainly by gastrointestinal events. We analyzed the effects of nintedanib on categorical changes in FVC in the subgroup of patients with autoimmune disease-related ILDs.
Methods: The INBUILD trial enrolled patients with a fibrosing ILD other than idiopathic pulmonary fibrosis, reticular abnormality with traction bronchiectasis (with or without honeycombing) of >10% extent on high-resolution computed tomography (HRCT), FVC ≥45% predicted, and DLco ≥30%–< 80% predicted. To be eligible to participate, patients had to meet protocol-specified criteria for progression of ILD within the 24 months before screening despite management as deemed appropriate in clinical practice. In the subgroup of patients with autoimmune disease-related ILDs, we analyzed absolute changes from baseline in FVC (mL) and FVC % predicted at week 52, and the proportions of patients with categorical declines in FVC % predicted at week 52 (analyzed using a worst observation carried forward approach).
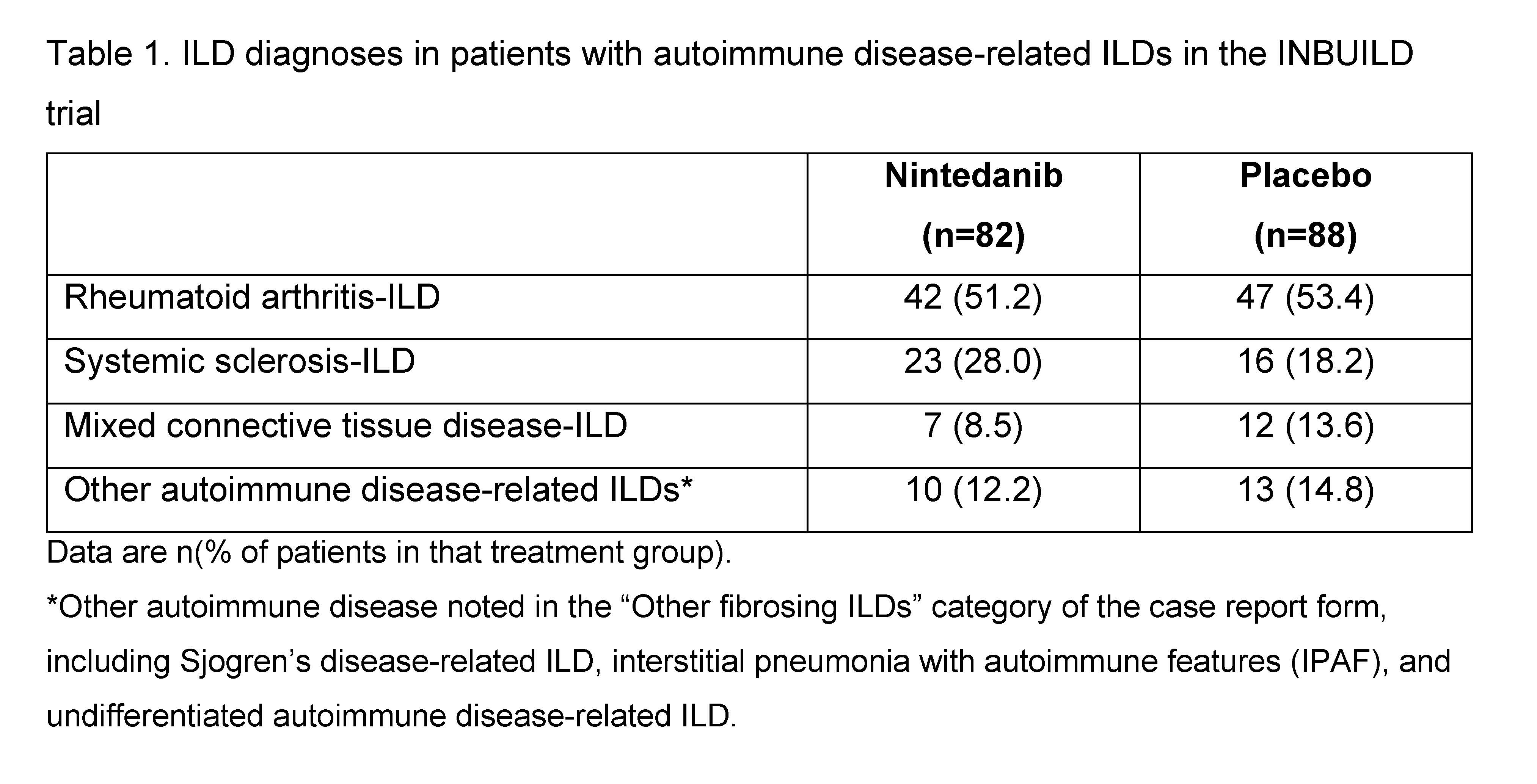
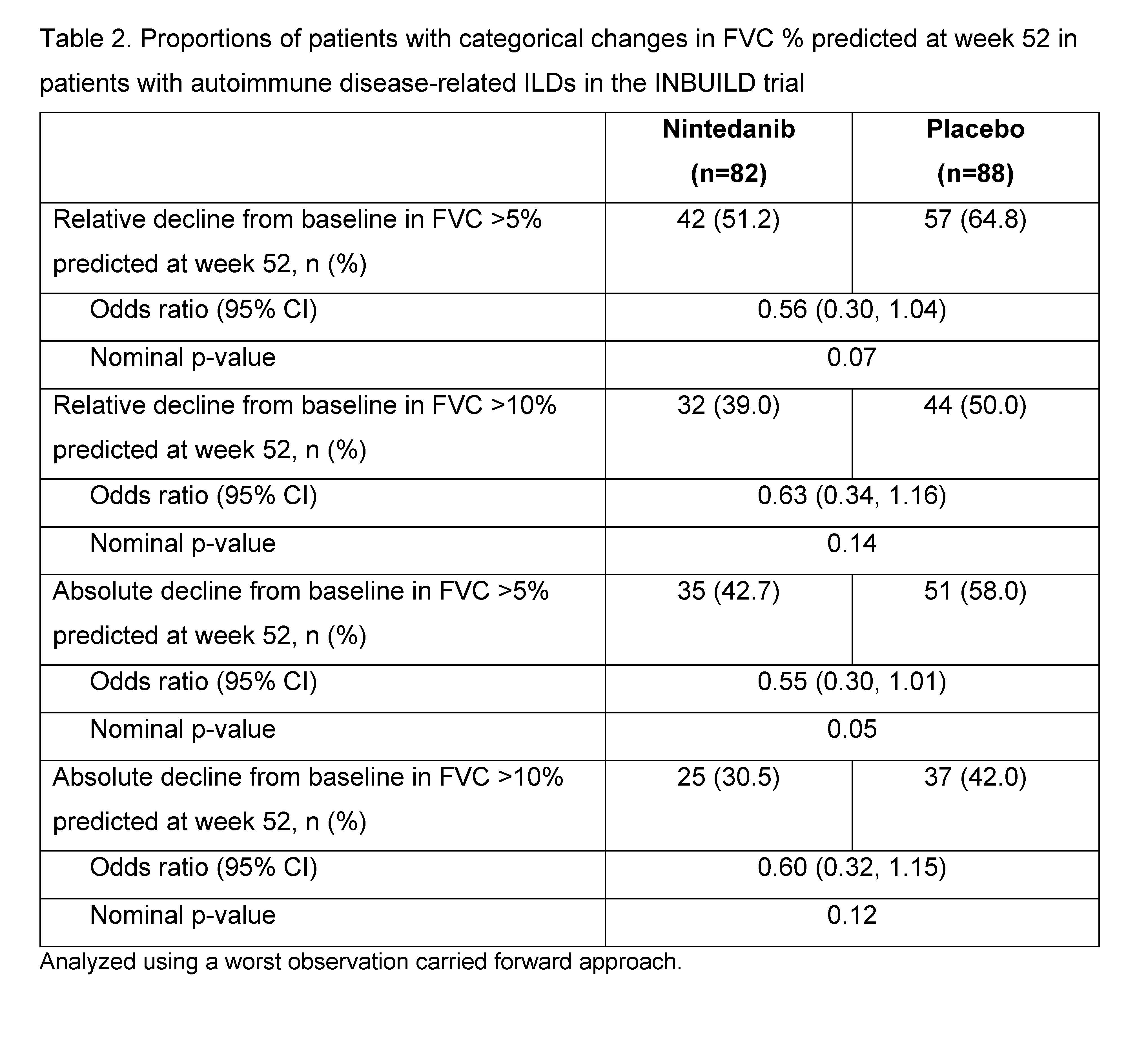
Results: The subgroup with autoimmune disease-related ILDs comprised 170 patients (Table 1). At baseline, mean (SD) FVC was 69.6 (15.1) % predicted in the nintedanib group and 72.1 (14.6) % predicted in the placebo group. Mean (SE) absolute changes from baseline in FVC were -92.7 (29.1) mL in the nintedanib group and -185.7 (27.3) mL in the placebo group (difference 93.0 [95% CI 14.1, 172.0]). Mean (SE) absolute changes from baseline in FVC % predicted were -2.7 (0.9) in the nintedanib group and -6.0 (0.8) in the placebo group (difference 3.3 [95% CI 0.9, 5.6]). The proportions of patients with absolute and relative declines in FVC >5% predicted or >10% predicted at week 52 were numerically lower in the nintedanib group than in the placebo group (Table 2).
Conclusion: In patients with progressive fibrosing autoimmune disease-related ILDs, the proportions of patients with categorical declines in FVC % predicted supported an effect of nintedanib in slowing the progression of ILD.
To cite this abstract in AMA style:
Matteson E, Distler O, Distler J, Kuwana M, Pope J, Seibold J, James A, Schlenker-Herceg R, Rohr K, Flaherty K. Reduced Decline in Forced Vital Capacity in Patients with Progressive Fibrosing Autoimmune Disease-Related Interstitial Lung Diseases (ILDs) Treated with Nintedanib [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/reduced-decline-in-forced-vital-capacity-in-patients-with-progressive-fibrosing-autoimmune-disease-related-interstitial-lung-diseases-ilds-treated-with-nintedanib/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reduced-decline-in-forced-vital-capacity-in-patients-with-progressive-fibrosing-autoimmune-disease-related-interstitial-lung-diseases-ilds-treated-with-nintedanib/