Session Information
Date: Sunday, October 26, 2025
Title: Plenary I (0772–0776)
Session Type: Plenary Session
Session Time: 9:45AM-10:00AM
Background/Purpose: Current ACR, EULAR and KDIGO guidelines recommend kidney biopsy in SLE patients with urine protein to creatinine (UPCR) ratio of >= 0.50 g/g. However, SLE patients who undergo kidney biopsy at lower levels of UPCR are frequently found to have proliferative lupus nephritis (Wang S, et al. Clin J Am Soc Nephrol. 2022;17:1150-1158). As part of the Accelerated Medicines Partnership we investigated first onset of UPCR 0.250-0.499 g/g.
Methods: SLE patients with no history of LN and UPCR of 0.250 to 0.499 g/g PLUS another predictor of lupus nephritis (LN) including non-White race, serologies (low C3, low C4, or anti-dsDNA). or active urine sediment, were enrolled. Kidney biopsies were done at the site institution and reported as ISN Class I-VI with NIH Activity and Chronicity Index, if LN.
Results: Twenty-eight patients were enrolled: 24 (86%) females, 6 (21%) Hispanic, and 13 (46%) African-American, 7 (25%) White, 2 (7%) Asian and 6 (21%) Other race. Twenty-five (89%) were anti-dsDNA positive, 20 (71%) had low C3 and 19 (68%) had low C4 at enrollment. All had GFR >60, no immunosuppression and prednisone < 10mg/day. Seven patients were taking ACEi/ARBs, two SGLT2 inhibitors (both were no LN on subsequent biopsy) and 23 hydroxychloroquine. Twenty had LN: two Class I; five Class II; six Class III; and seven Class V. Eight did not have LN (Table 3): 6 (75%) had a history of positive anti-dsDNA; 3 (38%) had a history of low C3; and 3 (38%) had a history of low C4. Table 1 shows the NIH Activity and Chronicity Indices, UPCR and serologies by ISN Class. There were no biopsy complications. Patients with LN were more likely to have had a history of low C3 (p=0.0223) or low C4 (p=0.0296) prior to enrollment (Table 2).In longitudinal follow up, two Class II, two Class III and three Class V have progressed to greater than 0.5 g/g of UPCR (in spite of treatment of Class III and Class V).
Conclusion: The 0.50 g/g UPCR cut-off for kidney biopsy in LN guidelines is arbitrary, omitting an opportunity to identify early LN before accrual of kidney damage. Earlier biopsy of LN, as defined by the AMP algorithm (UPCR between 0.250 and 0.499 g/g PLUS another LN predictor), found LN in 69%, with 61% initiating mycophenolate (6 Class III and 5 Class V) and 17% already showing some degree of chronicity. History of low C3 or C4 was the most important component of the AMP algorithm. The AMP early LN algorithm identified a high incidence of patients with consequential pathology and strongly support an adjustment to the UPCR cutoff for kidney biopsy in LN guidelines.
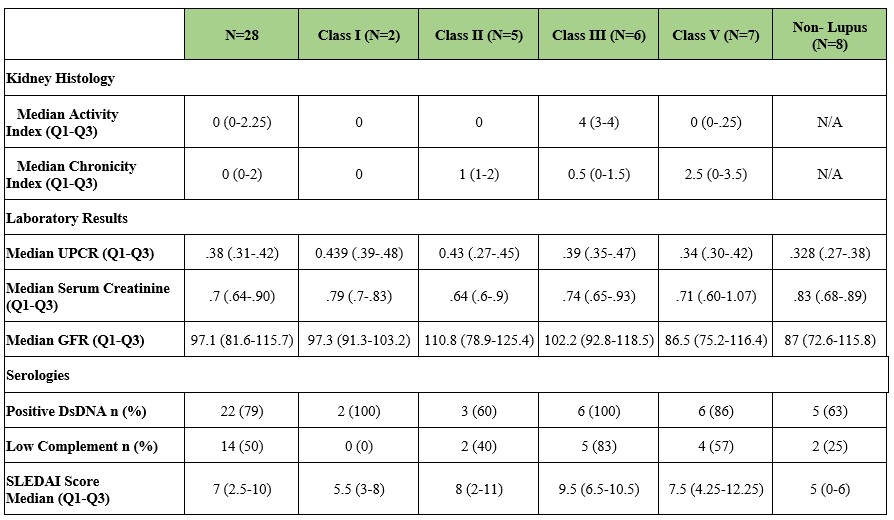 Table 1. Characteristics of lupus nephritis patients and biopsies
Table 1. Characteristics of lupus nephritis patients and biopsies
.jpg) Table 2. Comparison of inclusion criteria between those with lupus nephritis vs those without lupus nephritis
Table 2. Comparison of inclusion criteria between those with lupus nephritis vs those without lupus nephritis
.jpg) Table 3. Histology of non-lupus nephritis biopsies
Table 3. Histology of non-lupus nephritis biopsies
To cite this abstract in AMA style:
Petri M, Fava A, Atta M, Rosenberg A, Sanyal S, Izmirly P, Carter E, Masson M, Belmont M, Barnas J, Anolik J, Rovin B, Buyon J. Redefining When to Biopsy the Kidney in Patients with SLE [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/redefining-when-to-biopsy-the-kidney-in-patients-with-sle/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/redefining-when-to-biopsy-the-kidney-in-patients-with-sle/
