Session Information
Date: Monday, November 8, 2021
Title: RA – Treatments Poster II: PROs, Biomarkers, & Systemic Inflammation (1223–1256)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Accurately identifying patients who may respond better to a specific drug or mechanism of action could improve rational selection of medications to optimize clinical response, reduce irreversible damage, and ameliorate long term outcomes. Using a machine learning approach, previous work using data from clinical trials has found that patients with positive ACPA status (ACPA+) and CRP >12.3 mg/L responded better to sarilumab than patients who did not meet this criterion. However, the average disease activity and CRP levels for most patients initiating sarilumab in real-world settings is often lower than in clinical trials. Therefore, we validated the stratification rule in a national real-world rheumatology based electronic health record (EHR) registry. We hypothesized that sarilumab initiators who are rule-positive (seropositive [RF, ACPA] with CRP levels >12.3 mg/L) are more likely to achieve treatment targets compared with rule-negative patients.
Methods: Using 2017–2020 ACR’s Rheumatology Informatics System for Effectiveness (RISE) registry, we identified sarilumab initiators who had ≥1 prescription of sarilumab on or after 5/22/2017 (FDA approval date). The first prescription date was the index date. Eligible patients were required to be 18 years of age, ≥1 rheumatologist diagnosis for RA prior to index, clinical disease activity index (CDAI) >10 within 30 days prior to or 7 days after index, with CRP value within 90 days prior to index, and ≥1 CDAI within 6 ± 3 months after index. Based on the CDAI and CRP levels at the index date, we categorized the cohort into 4 groups, 1) Seropositive and CRP >12.3 mg/L, 2) Seropositive and CRP ≤12.3 mg/L, 3) Seronegative and CRP >12.3 mg/L, 4) Seronegative and CRP ≤12.3 mg/L. Logistic regression models were used to compare the odds of achieving CDAI low disease activity (LDA) or Remission (CDAI < 10), and MCID (minimum clinical important difference; CDAI improvement ≥12 if baseline CDAI >22 or CDAI ≥6 if baseline 10< CDAI < 22) between rule positive and negative patients. Sensitivity analyses using CRP >5 mg/L (rather than 12.3 mg/L) as the rule positive cutoff point were also conducted.
Results: We identified 205 sarilumab users who met all required inclusion and exclusion criteria. Baseline demographic and clinical characteristics are shown in Table 1; most characteristics of these patients were equally distributed across the four groups, except that seropositive patients were more likely to be of black race and less likely to have previous Janus kinase inhibitor use. Logistic regression showed that compared with rule negative patients, rule positive patients were more likely to reach LDA/remission and MCID (Table 2). The mean difference in Δ CDAI between rule positive and rule negative patients was 1–4 units (p=0.16). Results from sensitivity analyses (CRP >5 mg/L) yielded a better response in rule positive patients as in the main analysis.
Conclusion: Patients with seropositive status and elevated CRP ( >12.3 mg/L, or >5 mg/L) had a better response to sarilumab compared with patients who did not meet these two criteria in real-world settings. Seropositivity seemed to be a stronger driver for response in this data set and validation of an optimal CRP cutoff may require more data.
 Table 1: Baseline Demographic and Clinical Characteristics
Table 1: Baseline Demographic and Clinical Characteristics
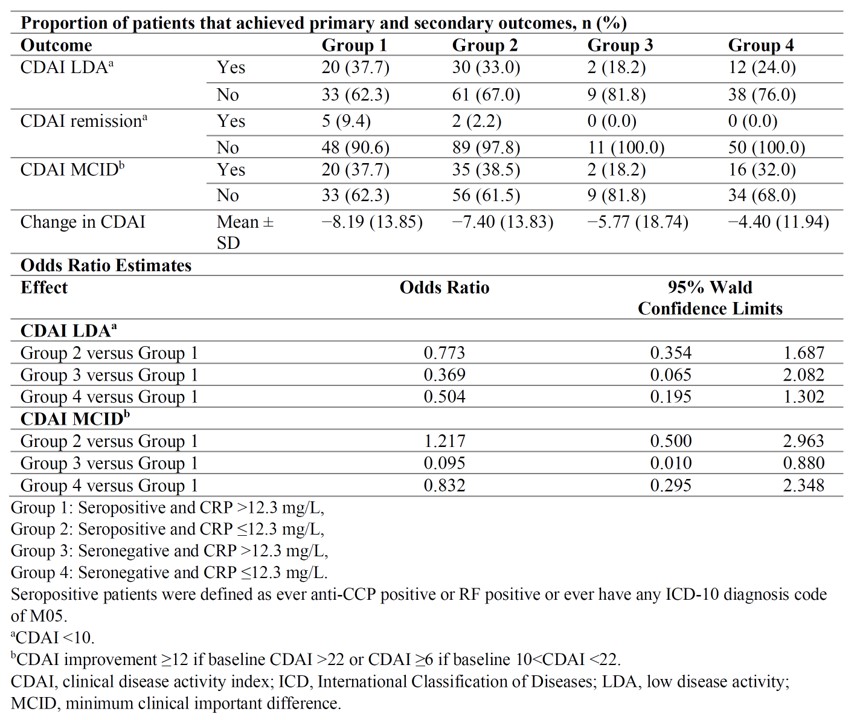 Table 2: Outcomes at 24 Weeks in Sarilumab Treated Patients
Table 2: Outcomes at 24 Weeks in Sarilumab Treated Patients
To cite this abstract in AMA style:
Yun H, Curtis J, Chen L, Clinton c, van Hoogstraten H, Fiore S, Rehberg M, Choy E. Real World Validation of a Rule to Predict Response to Sarilumab in Patients with Rheumatoid Arthritis: Analysis from the RISE Registry [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/real-world-validation-of-a-rule-to-predict-response-to-sarilumab-in-patients-with-rheumatoid-arthritis-analysis-from-the-rise-registry/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-validation-of-a-rule-to-predict-response-to-sarilumab-in-patients-with-rheumatoid-arthritis-analysis-from-the-rise-registry/
