Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The landmark ADVOCATE trial, which led to the approval of avacopan (AVP) as adjunctive treatment of ANCA-associated vasculitis (AAV), treated patients with AVP for 52 weeks. In real-world use, some patients are prescribed AVP for a longer duration. In this study, we describe a multi-center experience of prolonged courses of AVP including the outcomes at 52 weeks and adverse effects of AVP treatment beyond 52 weeks.
Methods: We performed a multi-center retrospective cohort study of 15 adult patients with new and relapsing AAV treated with AVP for a duration of 56 weeks or longer.
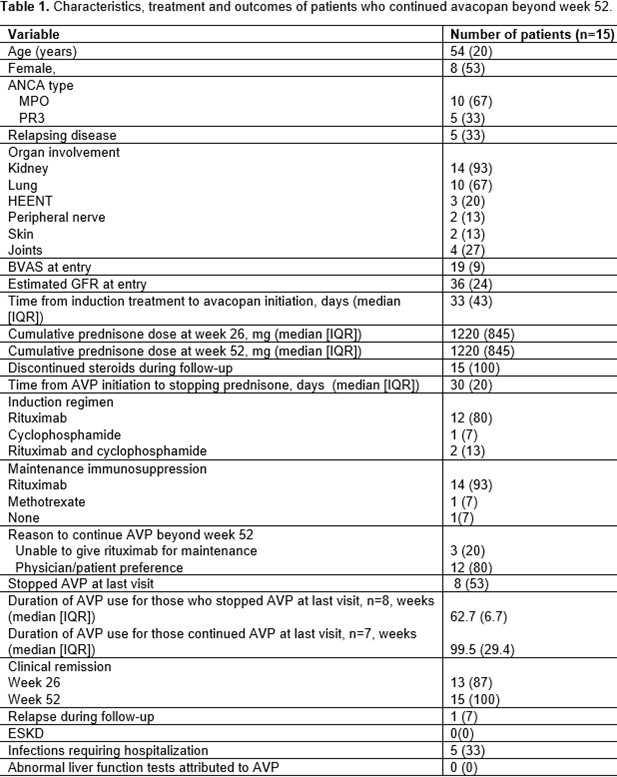
Results: Mean age was 54 (20) years, 53% were female, 10/15 were MPO-AAV, 5/15 were PR3-AAV, 14/15 had renal involvement and 5/15 had relapsing AAV. Mean follow-up was 96 (21) weeks. Rituximab was the preferred induction (12/15) and maintenance (14/15) therapy. During the initial 52 weeks AVP treatment, all patients discontinued prednisone within the first 26 weeks, mean eGFR rose by 15 and 18 mL/min/1.73m2 at 26 and 52 weeks respectively. Remission was achieved in 13/15 at week 26 and 15/15 at week 52. Reasons cited for treating with AVP beyond 52 weeks were physician and/or patient preference in 12 (80%) patients, and inability to give rituximab maintenance in 3 (20%) patients. 8/15 stopped AVP after a median (IQR) duration of 63 (7) weeks, 7/15 are still taking AVP for a median (IQR) duration of 99 (29) weeks when the data was compiled. Beyond 52 weeks, 3/15 could not be maintained on rituximab and AVP was used as maintenance. Outcomes are summarized in Table 1. During a mean follow-up period of 96 (21) weeks, 1/15 patients experienced AAV flare, and none progressed to ESKD. On last follow-up, the mean eGFR rise from baseline was 16 ml/min/1.73m2.Infections were the most commonly reported adverse effects including 5 infections requiring hospitalization. No patient had to discontinue AVP due to abnormal LFTs during the follow up period.
Conclusion: This real-world experience shows that AVP is being used in real-world practice beyond week 52, with patient/physician preference being the main reason. Rituximab was used alongside AVP for remission maintenance in majority of patients. Infection complications were the most observed adverse effects. Further data on the longer-term use of AVP is needed.
To cite this abstract in AMA style:
Ford J, Geara A, Geetha D, Singer O. Real-World Use of Avacopan for ANCA-Associated Vasculitis Beyond 52 Weeks [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/real-world-use-of-avacopan-for-anca-associated-vasculitis-beyond-52-weeks/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-use-of-avacopan-for-anca-associated-vasculitis-beyond-52-weeks/