Session Information
Date: Monday, November 13, 2023
Title: (1100–1123) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
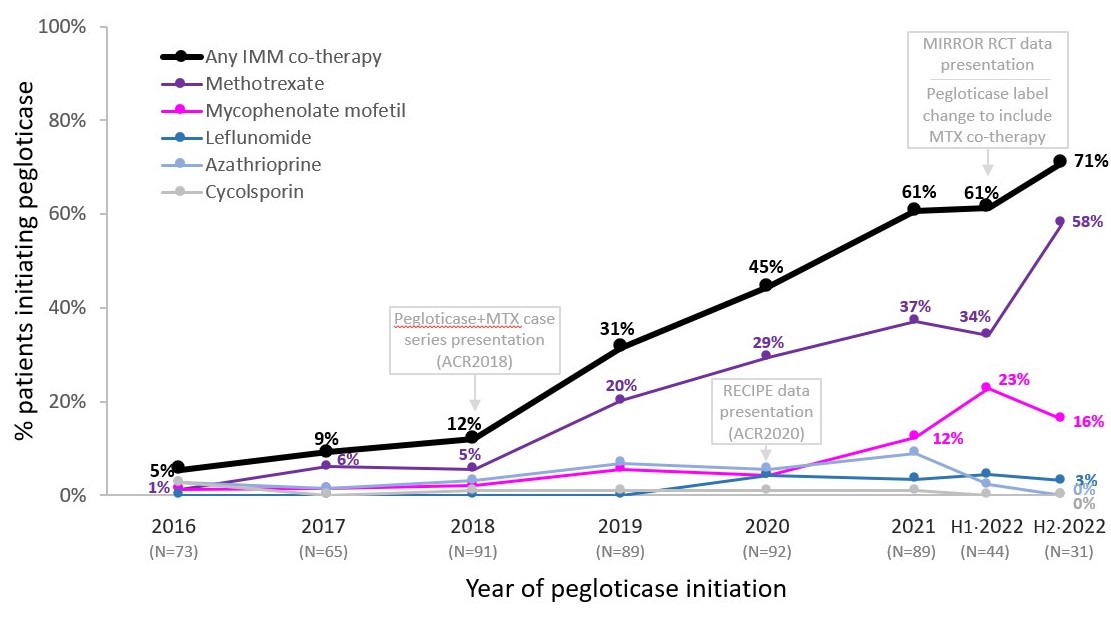
Background/Purpose: Pegloticase can treat uncontrolled gout but anti-drug antibodies limit urate-lowering response and put patients at risk for infusion reactions (IRs).1, 2 The first case series of pegloticase+methotrexate (MTX) co-administration was presented in Nov 2018, showing a 100% response rate with no IRs.3, 4 Real-world5 and clinical trial data6-8 have continued to support pegloticase+immunomodulation (IMM) co-therapy, with MIRROR RCT confirming pegloticase+MTX superiority (month 6 response rate: 71% vs. 39%; IR rate: 4% vs. 31%).9 The pegloticase label was updated to include MTX co-therapy in July 2022.10 Here, we examine real-world IMM co-therapy adoption through Nov 2022 using a large insurance claims database.
Methods: The Merative™ MarketScan® Research Databases (closed claims data, commercially-insured patients) were searched for patients with ≥1 pegloticase infusion code (J2507) between Jan 2016 and Nov 2022. Patients who had data within 180 days before and within 60 days after first J-code were included (not all patients who received pegloticase included). The use of IMM co-therapy (≥1 pharmacy claim [based on NDC code] for MTX, mycophenolate mofetil [MMF], azathioprine, leflunomide and/or cyclosporin ≤60 days before/after first J-code) was examined. Data are presented as mean(±SD) or n(%) as appropriate.
Results: 574 patients (91% men, 53.0±11.5 years old) met inclusion criteria. Diagnosis codes for hypertension, CKD, and cardiovascular disease were identified in 73%, 38%, and 32% of patients. Few patients (≤12%) received IMM co-therapy through 2018, but rates began to rise in 2019, presumably due to initial pegloticase+MTX case series (MTX use: 5% in 2018, 20% in 2019) and subsequent pegloticase+IMM data presentations; Figure). The proportion receiving IMM co-therapy continued to rise through 2022, with notable increases in MMF use following RECIPE presentation in Nov 2020 (ACR) and MTX use following MIRROR RCT presentation in Jun 2022 (EULAR) and pegloticase label update to include MTX in Jul 2022. After 2018, the majority of patients receiving IMM co-therapy had received MTX, followed by MMF and leflunomide (Figure).
Conclusion: Compelling real-world5, 11 and clinical trial9 efficacy/safety data has led to wide-spread awareness and adoption of IMM co-administration with pegloticase, often a last-hope therapy for patients suffering from uncontrolled gout. In the last part of 2022, over 70% of patients beginning pegloticase were co-administered IMM, with the majority of these patients receiving MTX.
REFERENCES 1. Sundy JS et al. JAMA 2011, 306:711-20 2. Lipsky PE et al. Arthritis Res Ther 2014;16:R60 3. Botson J, Peterson J. Arthritis Rheumatol 2018;70(supp 9) 4. Botson JK, Peterson J. J Clin Rheumatol 2022;28:e129-e34 5. Keenan RT et al. Semin Arthritis Rheum 2021;51:347-52 6. Botson JK et al. J Rheumatol 2021;48:767-74 7. Khanna P et al. Arthritis Rheum 2020;72(supp 10) 8. Rainey H et al. Ann Rheum Dis 2020;79(suppl 1):438 9. Botson JK et al. Arthritis Rheumatol 2023;75:293-304 10. Horizon Therapeutics USA. Pegloticase package insert 2022 11. Peterson J et al. Semin Arthritis Rheum 2021;51:1386-8 12. Khanna PP et al. Arthritis Rheumatol 2021;73:1523-32
To cite this abstract in AMA style:
Botson J, Fu Q, Zhu K, Padnick-Silver L, LaMoreaux B. Real-world Trends in the Use of Immunomodulation as Co-therapy to Pegloticase: Claims-based Findings Since 2016 [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/real-world-trends-in-the-use-of-immunomodulation-as-co-therapy-to-pegloticase-claims-based-findings-since-2016/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-trends-in-the-use-of-immunomodulation-as-co-therapy-to-pegloticase-claims-based-findings-since-2016/