Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The American College of Rheumatology (ACR) and American College of Chest Physicians (CHEST) recently released clinical practice guidelines for the treatment of RA-interstitial lung disease (RA-ILD). Given low certainty evidence, all recommendations were conditional in strength. There is a poor understanding of how the therapies included in the guideline have been used in real-world settings. In this study, we evaluated real-world use of these therapies for patients with RA-ILD within the Veterans Health Administration (VA).
Methods: We performed a cohort study of RA-ILD treatment patterns in the VA between 2006 and 2021. RA-ILD was identified using validated administrative algorithms. The initial encounter date with a diagnostic code for ILD was considered the RA-ILD diagnosis date. Pharmacy databases were queried for dispensing of medications any time before and after RA-ILD diagnosis. Patient demographics, comorbidities, RA-related measures, and ILD-related measures were obtained from VA databases. The primary comparison was the proportion of patients ever prescribed each medication before and after RA-ILD diagnosis using McNemar’s tests. Sensitivity analyses were performed for RA-ILD diagnoses occurring in a more recent treatment period (2012 to 2021). Among patients not using a medication recommended for ILD treatment at the time of RA-ILD diagnosis, we evaluated predictors of initiation using multivariable logistic regression.
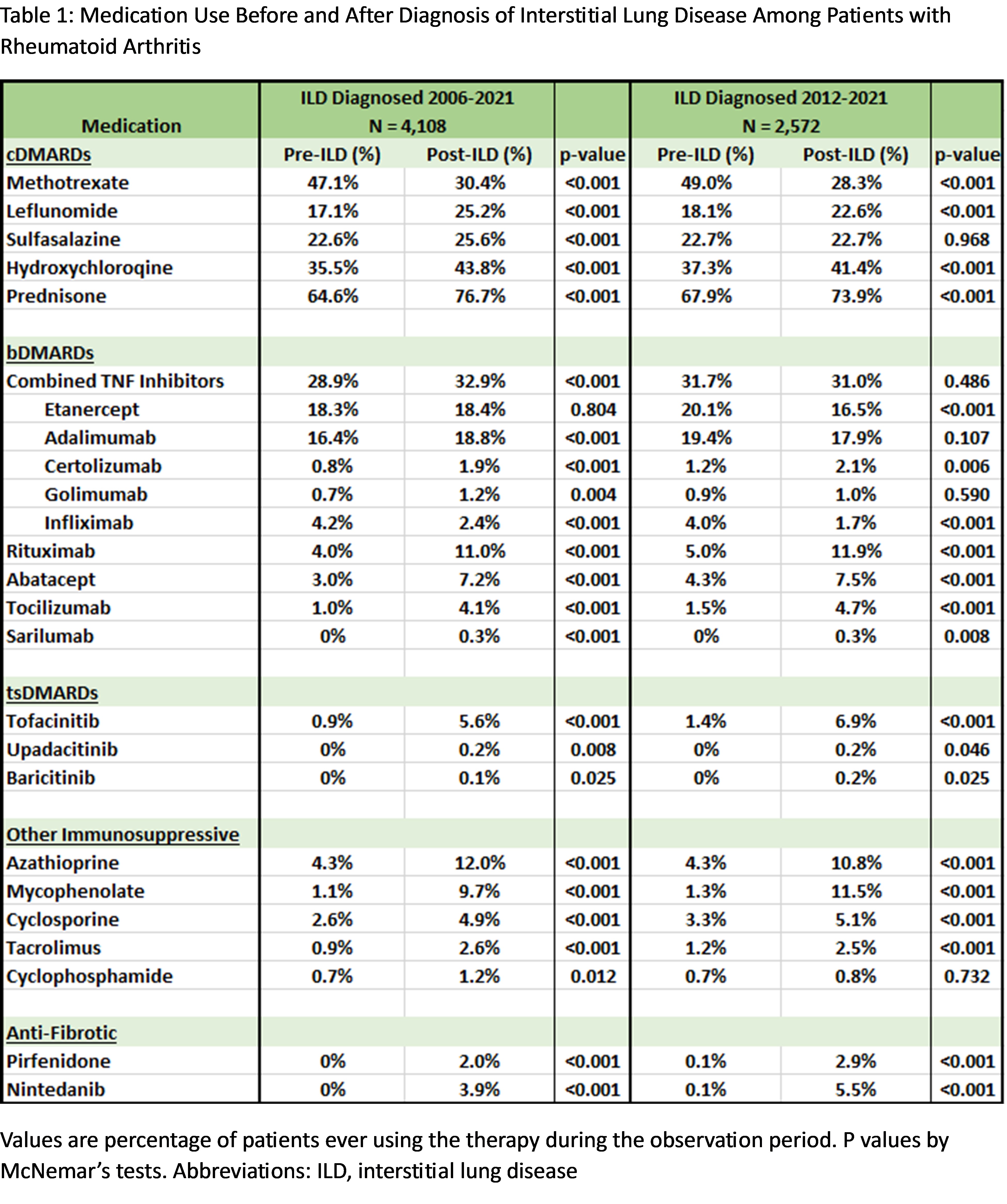
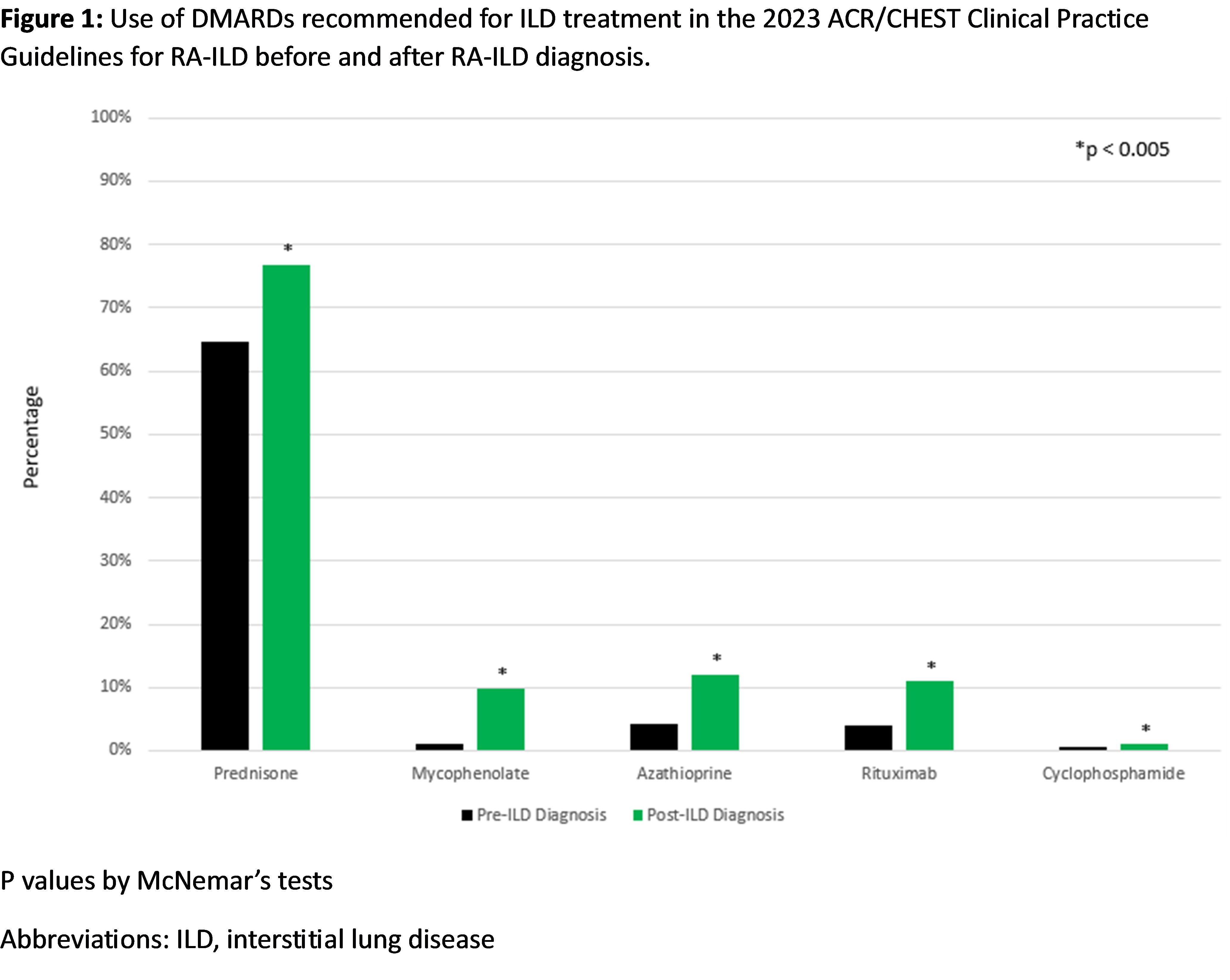
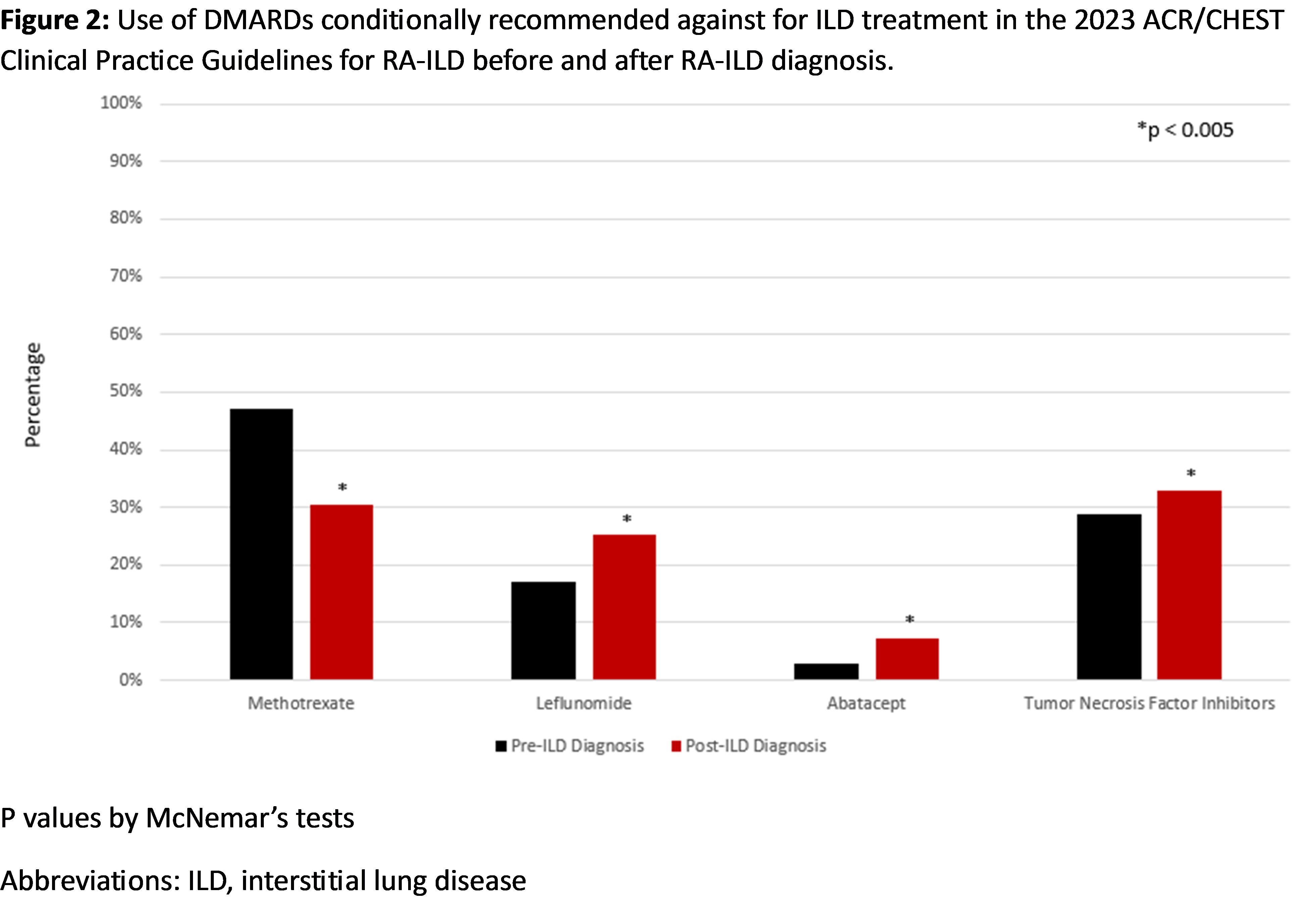
Results: We studied 4,108 RA-ILD patients (mean age 68.5 years, 93.4% male). Among the medications recommended for ILD treatment, prednisone was commonly used before and after ILD diagnosis (Table 1, Figure 1). Mycophenolate, azathioprine, rituximab, and cyclophosphamide use increased after ILD diagnosis, but the percent of patients dispensed these therapies remained low (1.2% to 12.0%). Among the medications recommended against for ILD treatment, there was a significant decrease in methotrexate use (47.1% to 30.4%), while there was a significant increase in the use of leflunomide, tumor necrosis factor inhibitors (TNFi), and abatacept (Table 1, Figure 2). Among patients not receiving a recommended medication for ILD treatment at diagnosis, forced vital capacity (FVC) of 60-80% (vs. >80%) predicted (aOR 1.50 [1.21, 1.87]), FVC < 60% (vs. >80%) predicted (aOR 1.57 [1.21, 2.03]), anti-CCP/RF seropositivity (aOR 1.81 [1.42, 2.31]), and ILD diagnosis between 2012-2021 (aOR 1.44 [1.22, 1.71]) were associated with subsequent initiation. Older age at RA-ILD diagnosis, higher comorbidity burden, and longer RA duration were associated with less frequent initiation.
Conclusion: Most first-line therapies conditionally recommended for ILD treatment in the ACR/CHEST clinical practice guideline were infrequently used in real-world settings. These findings help contextualize the guideline, highlight the heterogeneity of RA-ILD, and suggest that treatment decisions in RA-ILD are guided by several patient factors, including those beyond ILD severity. Further research is needed to evaluate treatment patterns in specific RA-ILD populations, including individuals with a progressive course.
To cite this abstract in AMA style:
Dunn J, Matson S, George M, Yang Y, Roul P, Rojas J, Cannon g, brian S, Curtis J, Baker J, Mikuls T, England B. Real-World Treatment Patterns of Clinical Practice Guideline Therapies in Rheumatoid Arthritis-Associated Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/real-world-treatment-patterns-of-clinical-practice-guideline-therapies-in-rheumatoid-arthritis-associated-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-treatment-patterns-of-clinical-practice-guideline-therapies-in-rheumatoid-arthritis-associated-interstitial-lung-disease/