Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The international SLE Prospective Observational Cohort Study (SPOCS) collected data on patients with moderate to severe SLE disease activity from June 2017 through November 2022. A unique aim of SPOCS is to evaluate relationships between various aspects of SLE and type I IFN gene signature (IFNGS) status.
Objectives: To assess medication use among SPOCS patients over 36 months.
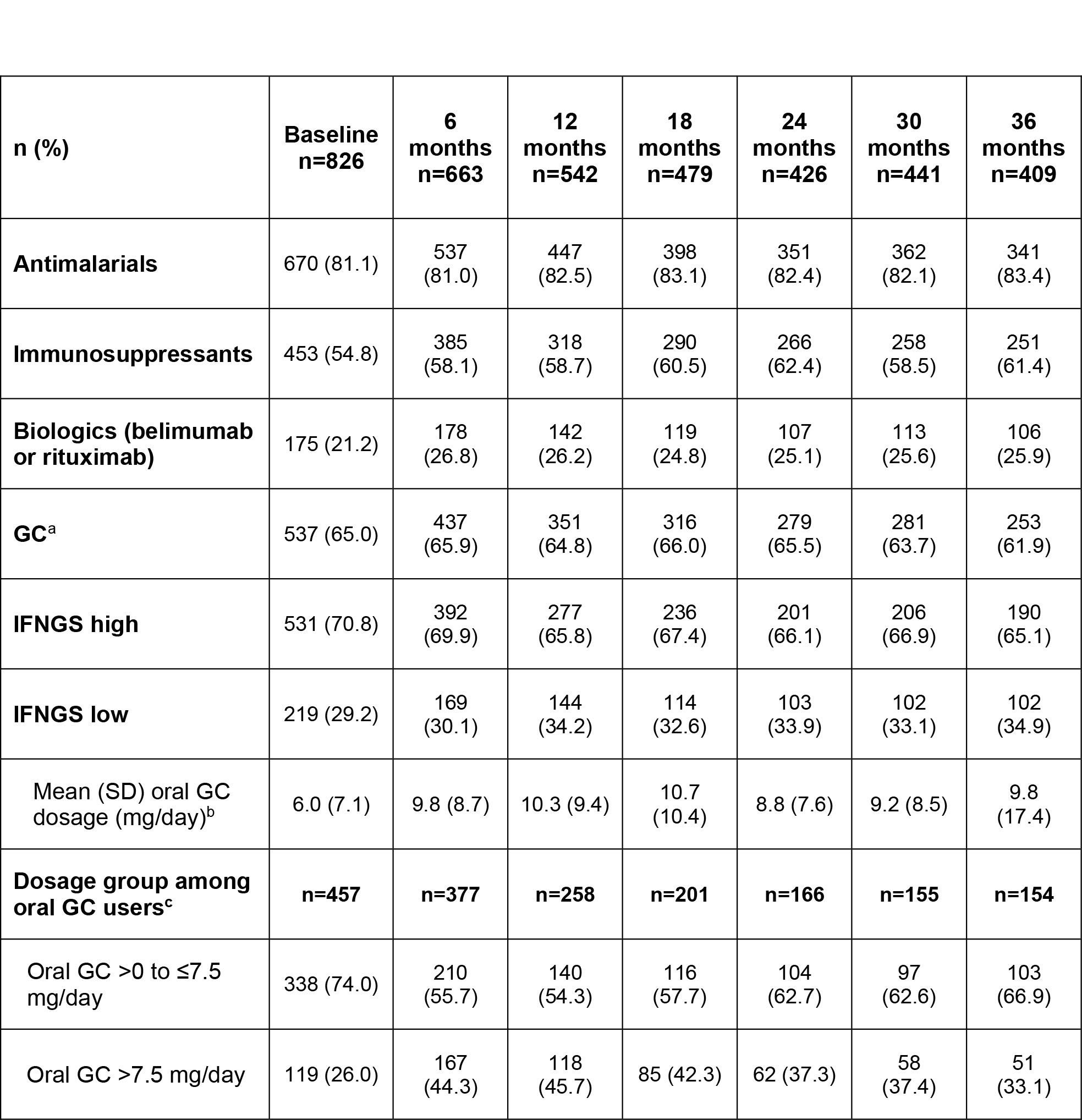
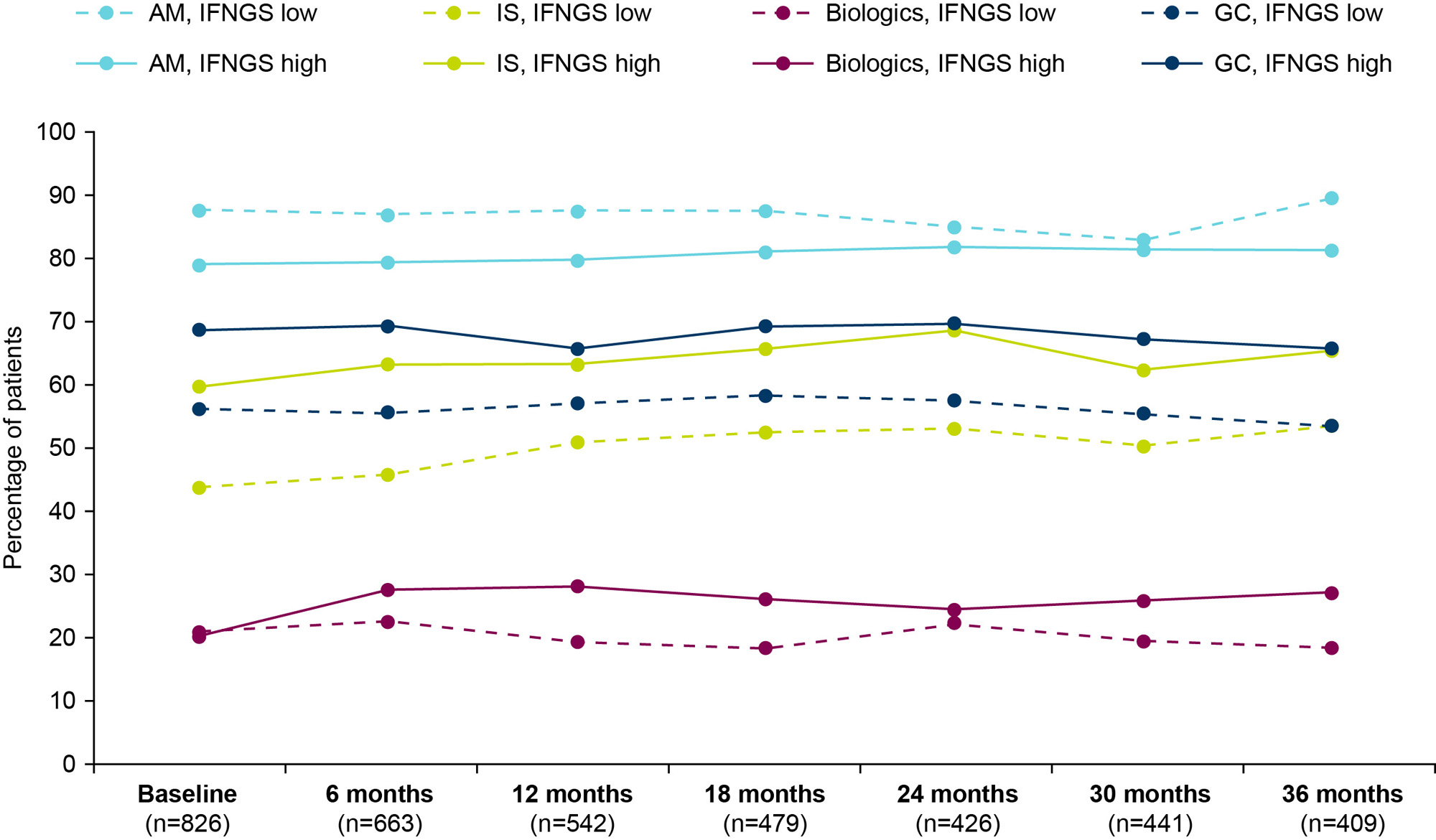
Methods: Patients enrolled in SPOCS were ≥18 years old with moderate to severe active SLE (SLEDAI ≥6 or clinical SLEDAI ≥4) and received standard therapy with ≥6 months of systemic SLE treatment beyond non-steroidal anti-inflammatory drugs and analgesics. All patients met ACR or Systemic Lupus International Collaborating Clinics (SLICC) SLE classification criteria. Medication use data were collected over 36 months during patients’ biannual clinic visits. In this analysis, we report the proportions of patients receiving antimalarials, immunosuppressants (IS), biologics (belimumab or rituximab), or glucocorticoids (GCs), as well as daily oral GC dosage (mg/day) and proportions of patients receiving low ( >0 to ≤7.5 mg/day) or high ( >7.5 mg/day) oral GC dosages. Medication use was also stratified by baseline IFNGS (high vs low).
Results: At baseline, most patients (n=826; IFNGS-high [70.8%], low [29.2%]) were receiving antimalarials (81.1%), 21.2% were receiving biologics, and 54.8% were receiving IS (Table). From baseline to 36 months, the proportions of patients taking antimalarials remained stable while the use of biologics and IS moderately increased. At baseline, 65.0% of patients were taking GCs and the proportion was largely stable over time. However, the proportion administered oral GC >7.5 mg/day increased from 26.0% at baseline to 44.3% at 6 months and 45.7% at 12 months, and then declined with time but did not reach the baseline level. The mean (SD) dose of oral GC increased from 6 (7.1) mg/day at baseline to 10 (8.7) mg/day at 6 months and then was stable over time. Across the study period, IS, biologics, and GCs were generally administered to greater proportions of IFNGS-high than low patients (Figure). Mean oral GC daily doses were similar between IFNGS-high and low groups throughout the study period except at the final visit. At 36 months, IFNGS-low patients received a lower mean (SD) oral GC daily dose compared with IFNGS-high patients (7 [4.9] vs 11 [20.6] mg/day).
Conclusion: Overall, the increase in oral mean daily GC dose in the first 6 months was maintained through 36 months despite standard therapy, stable antimalarial use, and moderate increases in use of biologics and IS. Although the proportion of GC use was largely stable over time, those on oral GC >7.5 mg/day increased initially and then declined but did not return to the baseline level. In addition, IFNGS-high status was associated with more immunomodulatory therapy compared with those with IFNGS-low status. Together, these data indicate that additional therapies are needed to avoid high-dose GC use among patients with SLE. SPOCS will provide opportunities to increase our understanding of real-world treatment and to identify educational needs within the lupus community.
To cite this abstract in AMA style:
Aringer M, Arnaud L, Furie R, Morand E, Peschken C, Desta B, Rapsomaniki E, Hedberg J, Grünfeld Eén T, Sorrentino A, Ghia C, Chen S, Ding B. Real-World Treatment Patterns in Patients with Systemic Lupus Erythematosus: An Analysis of the SLE Prospective Observational Cohort Study (SPOCS) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/real-world-treatment-patterns-in-patients-with-systemic-lupus-erythematosus-an-analysis-of-the-sle-prospective-observational-cohort-study-spocs/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-treatment-patterns-in-patients-with-systemic-lupus-erythematosus-an-analysis-of-the-sle-prospective-observational-cohort-study-spocs/