Session Information
Date: Saturday, November 12, 2022
Title: Health Services Research Poster I: Lupus, RA, Spondyloarthritis and More
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: SLE treatment is complex, with a wide variety of medications commonly prescribed. Limited evidence exists in the literature with respect to treatment patterns, HCRU and costs within different lines of therapy (LOT), especially for biologics. We aimed to describe the characteristics, treatment patterns, HCRU and associated costs, in adult patients with SLE in the US.
Methods: This retrospective observational cohort study used IBM MarketScan® Commercial and Medicare Supplemental Databases to identify patients aged ≥18 years, with a new diagnosis code for SLE without prior SLE treatment. Patients with a new SLE diagnostic code between January 2013 to December 2017 (index date), who had ≥24 months of continuous enrollment both prior to this date without an SLE medication (i.e., antimalarials, immunosuppressants, or biologics) and following this index date started a new SLE medication, were eligible for analysis. Date of the earliest prescription for SLE treatment, on or following the index date, was defined as LOT1. A new medication (added or switched to) resulted in a new LOT. Up to 3 LOTs were considered among SLE patients newly initiating SLE treatment during follow-up. For each LOT, medication use, HCRU, and healthcare costs attributable to SLE were reported.
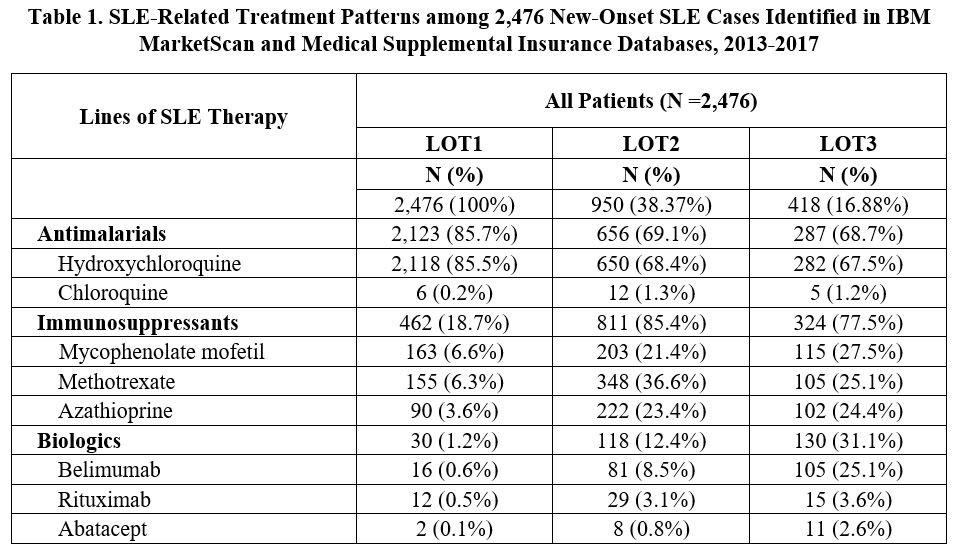
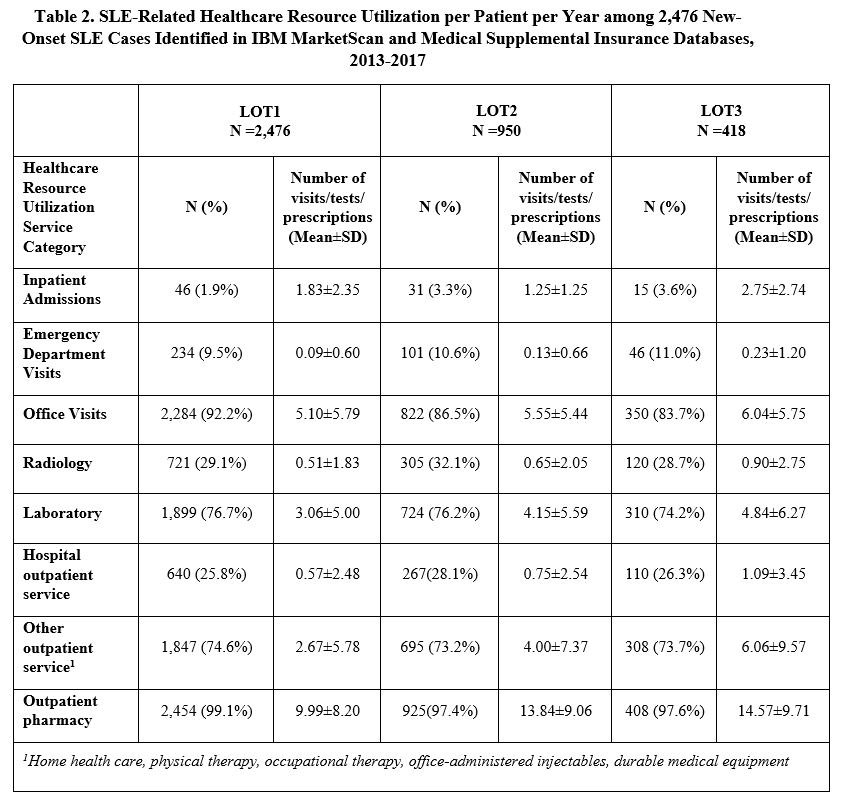
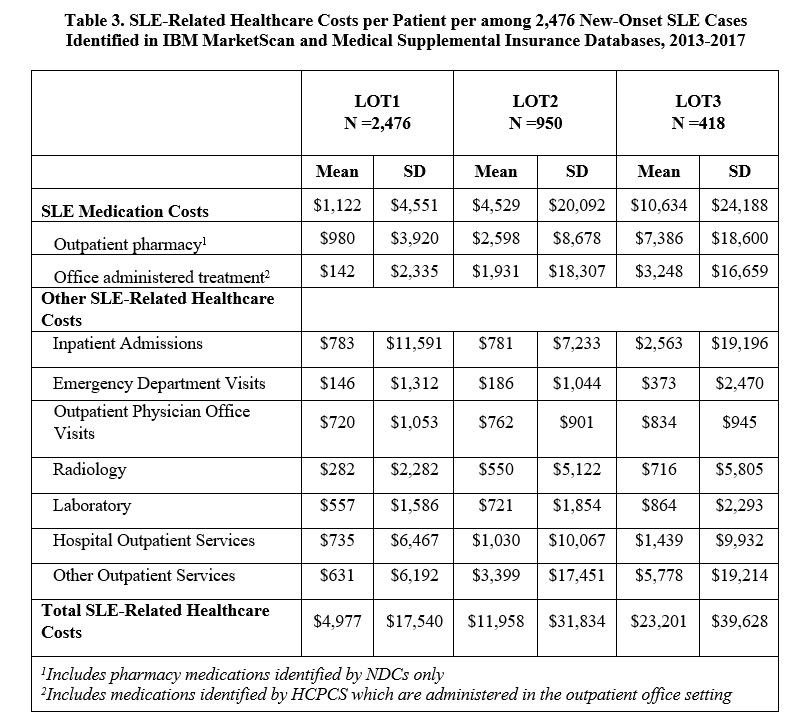
Results: A total of 2,476 incident SLE patients were identified and received a first LOT. The mean (SD) age was 46.9 (14.1) years, 86.9% were female, and follow-up duration was 1,435 (472) days. 950 (38.4%) progressed to LOT2, and 418 (16.9%) moved on to LOT3 (Table 1). Hydroxychloroquine was the most commonly used medication during LOT1 (85.5%), with immunosuppressants being most frequently used in LOT2 (85.4%) and LOT3 (77.5%). Among immunosuppressants, methotrexate (36.6%) was the most commonly used LOT2 drug. During LOT3, 31.1% of patients received biologics, with belimumab the most common (25.1%). The proportion of patients utilizing healthcare resources stayed consistent across LOTs by service category, while the mean number of visits/tests/prescriptions increased with LOT (Table 2). Mean (SD) annual total SLE medication cost per patient increased from $1,122 ($4,551) in LOT1 to $4,529 ($20,092) and $10,634 ($24,188) for LOT2 and LOT3, respectively (Table 3). Other SLE-related healthcare costs also increased from $3,855 in LOT1 to $7,429 and $12,567 in LOT2 and LOT3. The difference in total SLE-related healthcare costs from LOT1 to LOT3 was $18,224.
Conclusion: Among US adult SLE patients with commercial health insurance, we found a variety of medications prescribed by LOT among patients newly initiating treatment. The mean number of healthcare visits/tests/prescriptions and associated costs, increased with LOT, with approximately half of costs driven by healthcare costs other than medication. The results highlight the proportion of new onset SLE patients that progress to LOT3, with a consequent increase in HCRU and costs.
To cite this abstract in AMA style:
Masurkar P, Reckleff J, Princic N, Limone B, Schwartz H, Karis E, Zollars E, Stolshek B, Costenbader K. Real-World Treatment Patterns, Healthcare Resource Utilization (HCRU) and Costs in Patients with Systemic Lupus Erythematosus (SLE) in the US [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/real-world-treatment-patterns-healthcare-resource-utilization-hcru-and-costs-in-patients-with-systemic-lupus-erythematosus-sle-in-the-us/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-treatment-patterns-healthcare-resource-utilization-hcru-and-costs-in-patients-with-systemic-lupus-erythematosus-sle-in-the-us/