Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Dermatomyositis (DM) is a rare chronic systemic autoimmune disease characterized by muscle weakness, skin rashes, and elevated risk of associated conditions such as interstitial lung disease, calcinosis, dysphagia, cardiac complications, and malignancy. Therapeutic approaches for DM include corticosteroids (which can cause substantial toxicity with long-term use) and corticosteroid-sparing immunosuppressive therapy (IST). Limited data are available on current prescribing practices in DM. This study aimed to describe real-world treatment patterns in a prevalence cohort of adult patients with DM, including treatment discontinuation, switching, and concomitant corticosteroid use over time.
Methods: A retrospective cohort was identified within PharMetrics Plus (Jan 2014 – June 2020), a large administrative database of employer-sponsored health plans in the United States. The study included patients (age ≥18 years) who were diagnosed with DM (≥1 inpatient claim or ≥2 outpatient claims ≥30 days apart with International Classification of Diseases, 10th Revision: M33.0x, M33.1x, or M33.9x), initiated a DM-related treatment (in any treatment line), and were continuously enrolled in the health plan for 6 months before and 1 year after treatment initiation (index date). Outcomes were evaluated over the 1-year post-index period using Kaplan-Meier analysis, including time to index treatment discontinuation (≥60-day gap in supply) and switching (initiation of a different DM treatment < 60 days before or after index drug discontinuation). Among patients with concomitant corticosteroid use within 60 days of the index date, time from the index date to corticosteroid discontinuation was also examined to assess ongoing dependence on corticosteroids following index treatment initiation.
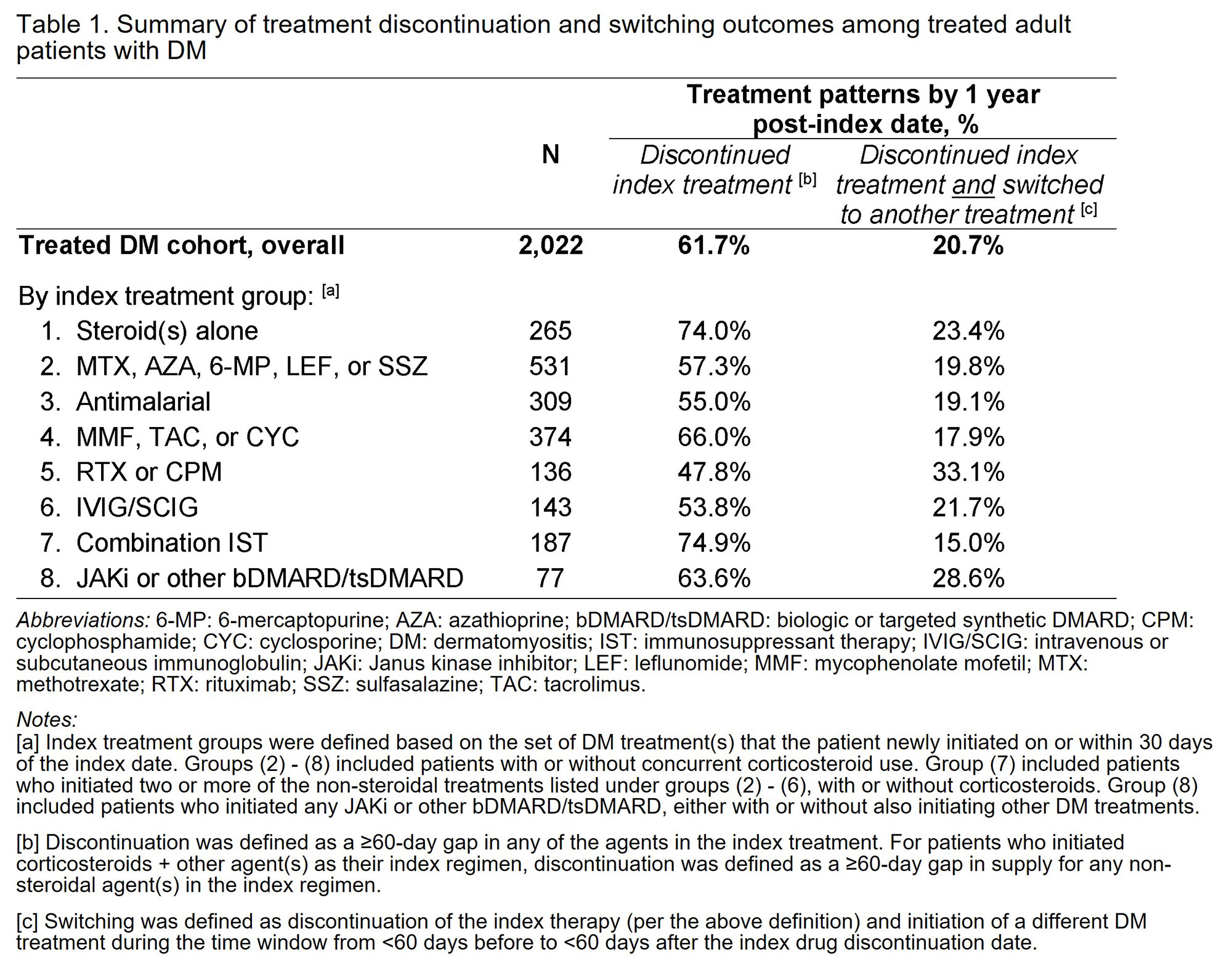
Results: Among treated patients with DM (N=2,022), 79.6% were female and mean age was 51.7 years. By 1 year post-index date, 61.7% of all patients discontinued the index treatment, and 20.7% of all patients (33.6% of discontinuers) switched to another DM treatment (Table 1; Figure 1). Across different index treatment groups, treatment discontinuation by 1 year ranged from 47.8% (for patients who initiated rituximab or cyclophosphamide) to 74.9% (for those who initiated combination IST). Treatment switching by 1 year ranged from 15.0% (for combination IST) to 33.1% (for rituximab or cyclophosphamide). Among patients who switched, the distribution of subsequent therapies varied depending on the index treatment (Figure 2). In groups that initiated ≥1 non-steroidal DM treatment as index therapy, 37.2% – 52.4% also used corticosteroids concomitantly; of these patients, 41.2% – 52.8% continued corticosteroids for the entire 1-year post-index period.
Conclusion: In a cohort of treated patients with DM, the majority discontinued their index treatment regimen within 1 year of initiation. Concurrent use of corticosteroids was common, with many patients remaining on corticosteroids 1 year after initiating a non-steroidal index treatment. Findings from this real-world claims database analysis suggest that additional treatment options for DM may be important.
To cite this abstract in AMA style:
Bensimon A, Chen K, Noman A, Yim E, Xenakis J, Aggarwal R. Real-World Treatment Patterns Among Adult Patients with Dermatomyositis in the United States [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/real-world-treatment-patterns-among-adult-patients-with-dermatomyositis-in-the-united-states/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-treatment-patterns-among-adult-patients-with-dermatomyositis-in-the-united-states/