Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Psoriatic arthritis (PsA) is a chronic musculoskeletal disease, which can lead to progressive joint pain, joint destruction, and loss of function. Evidence suggests that delayed diagnosis of even six to twelve months is associated with radiographic joint damage and poor functional outcomes in PsA.
To examine demographic and clinical characteristics associated with diagnostic delay in an inception PsA cohort.
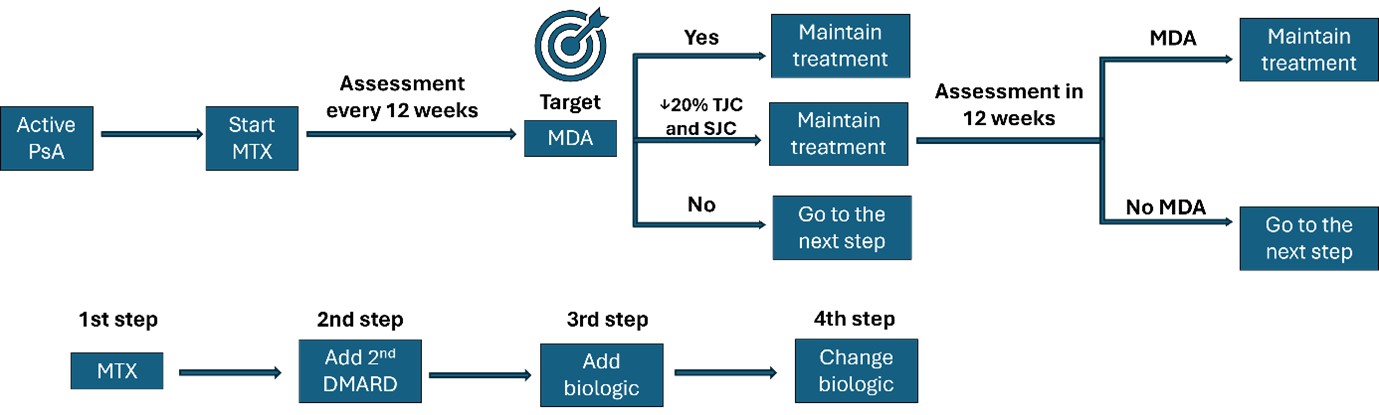
Methods: The Multicentre Observational Initiative in Treat to target Outcomes in Psoriatic Arthritis (MONITOR-PsA) study (NCT03531073) is a prospective, population-based cohort of adult PsA patients from 13 secondary care rheumatology centres across UK, between 2018–2024. All participants met CASPAR criteria and were treated routinely according to treat-to- target strategy (Fig 1).
The study collected clinical and patient-reported outcomes that align with the 2016 OMERACT core and strongly recommended domains for PsA. In addition, radiographic damage was determined based on joint erosion score, destruction and proliferation from hand/wrist and feet radiographs, and joint space narrowing (JSN) as included in the Sharp-van der Heijde modified (SvdH) score for PsA.
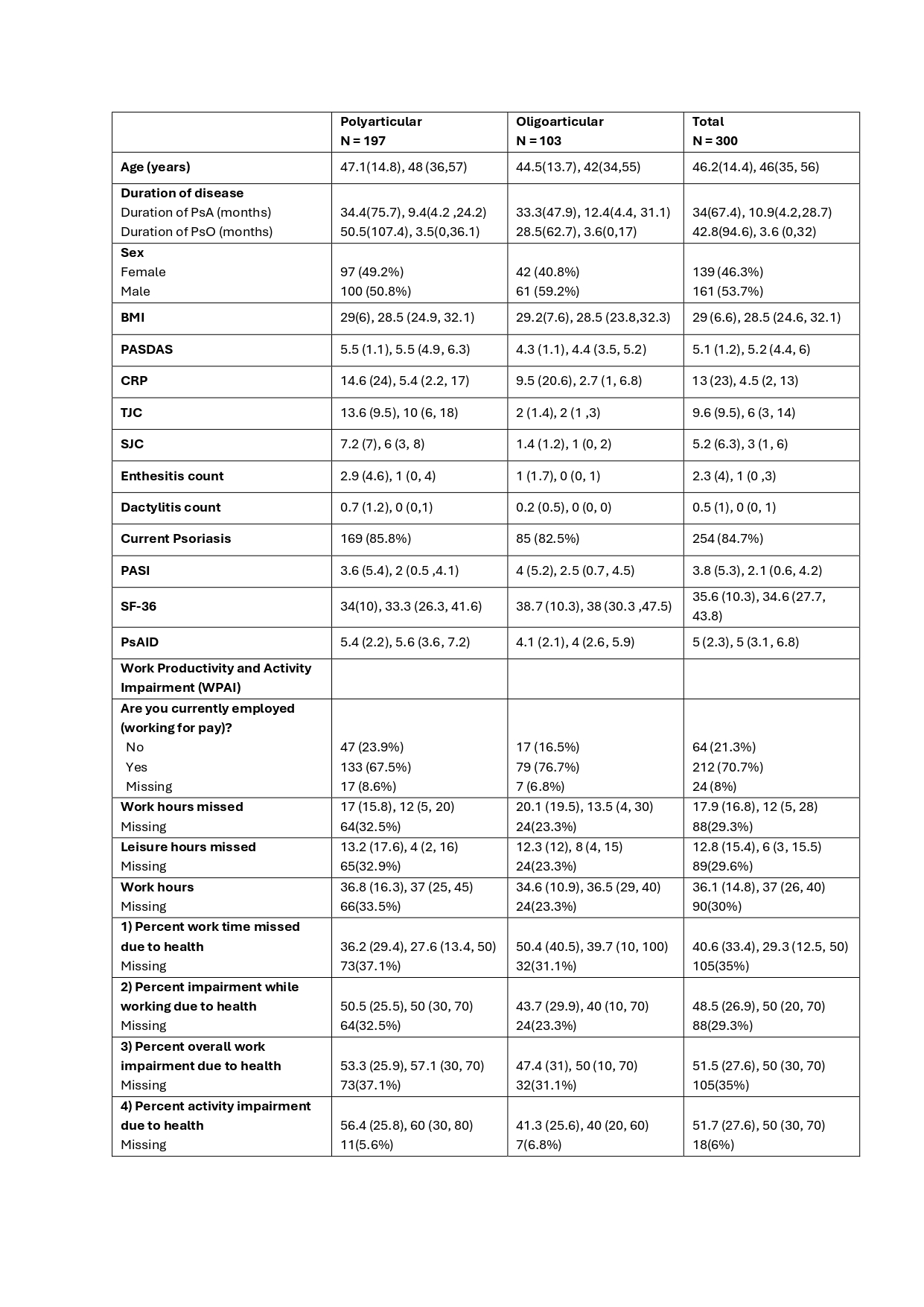
Results: Baseline data were available for 300 participants. 46.3% were female, and 63% had polyarticular arthritis (Table 1). The mean (SD) age was 46.2 (14.4) years, and the mean disease duration was 34 (67.4) months, with a median (IQR) of 10.9 (4.2, 28.7) months at diagnosis. The polyarthritis group (joint count >3, n=197) had a mean disease duration of 34.4 (75.7) months and the oligoarthritis group (n=103) mean duration of 33.3 (47.9) months.
The mean (SD) PASDAS score of 5.1 (1.2) indicated moderate disease activity (defined PASDAS score greater than 3.2 and less than 5.4) and mean (SD) PASI score of 3.8 (5.3). At the initial presentation, 48.3% had enthesitis, 24.3% had dactylitis and 63.7% reported inflammatory back pain lasting for more than 3 months with a mean (SD) BASDAI score of 5.6 (2.4). Additionally, there were observed cardio-metabolic comorbidities including: overweight (41.3%), elevated liver enzymes (32%), hypertension (16%), diabetes mellitus (7%), and dyslipidaemia (7%).
At the time of presentation, 25% of the participants had erosive disease, with 19.7% experiencing erosive disease in the hand joints and 10% in the feet joints. Among those with erosive disease, the mean (SD) Total SvdH score was 7.7 (11.2), with a mean (SD) erosion score of 1.4 (3.4) and a mean JSN score of 7.8 (9.3).
The mean (SD) HAQ disability score was 0.9 (0.7), indicating moderate to severe disability (moderate score 0.8 ≤ to < 1.2). The mean (SD) PsAID-12 score at baseline was 5.0 (2.3), with a PsAID score ≥4 indicating a high impact of PsA.
Conclusion: In this real-world inception cohort, it was observed that patients with PsA still experience significant delays in receiving a diagnosis. One-quarter of patients had radiographic damage at diagnosis, identical to the proportion in the 2003 Kane et al. Irish study. At the time of diagnosis, most patients experience a high disease burden, including radiographic damage, high disability scores, and a negative impact on their quality of life.
DMARD: Disease-Modifying Antirheumatic Drug, MTX: methotrexate, PsA: Psoriatic Arthritis
All the variables are presented as mean (SD), median (IQR). Sex, working for pay, and current psoriasis are presented as n (%).
SD: standard deviation, IQR: interquartile range
To cite this abstract in AMA style:
James L, Saeedi E, Letarouilly J, Gullick N, Francis A, Jadon D, Tillett W, Sinomati Y, Tucker L, Mian N, Rombach I, Marian I, Massa M, Coates L. Real-World Treat-to-Target Strategy in Psoriatic Arthritis: Baseline Characteristics from the MONITOR-PsA Cohort [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/real-world-treat-to-target-strategy-in-psoriatic-arthritis-baseline-characteristics-from-the-monitor-psa-cohort/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-treat-to-target-strategy-in-psoriatic-arthritis-baseline-characteristics-from-the-monitor-psa-cohort/