Session Information
Date: Saturday, November 12, 2022
Title: Health Services Research Poster I: Lupus, RA, Spondyloarthritis and More
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: There is a growing concern over the rise of polypharmacy, arguably one of the most pressing prescribing challenges. The option of biologic monotherapy is particularly vital for patients with rheumatoid arthritis (RA) who are also likely to receive treatment for other chronic conditions, such as osteoporosis. Hence, the aim of this nationwide population-based study was to provide real world evidence as regards biologic monotherapy for RA patients in Taiwan.
Methods: We identified a retrospective cohort of 6,991 patients who had at least one primary or secondary diagnosis of RA and received initial biologics treatments in outpatients or emergency services between January 1, 2015 and December 31, 2018 from the National Health Insurance Research Database in Taiwan. After excluding ineligible patients (patients who had active tuberculosis prior to the index date, received rituximab, baricitinib or certolizumab pegol as the index treatment, not received any csDMARD, aged below 18 years, or with missing data), ultimately, 5,805 patients were included in the study. We then analyzed incidence rates of biologic monotherapy treatment of those qualified patients.
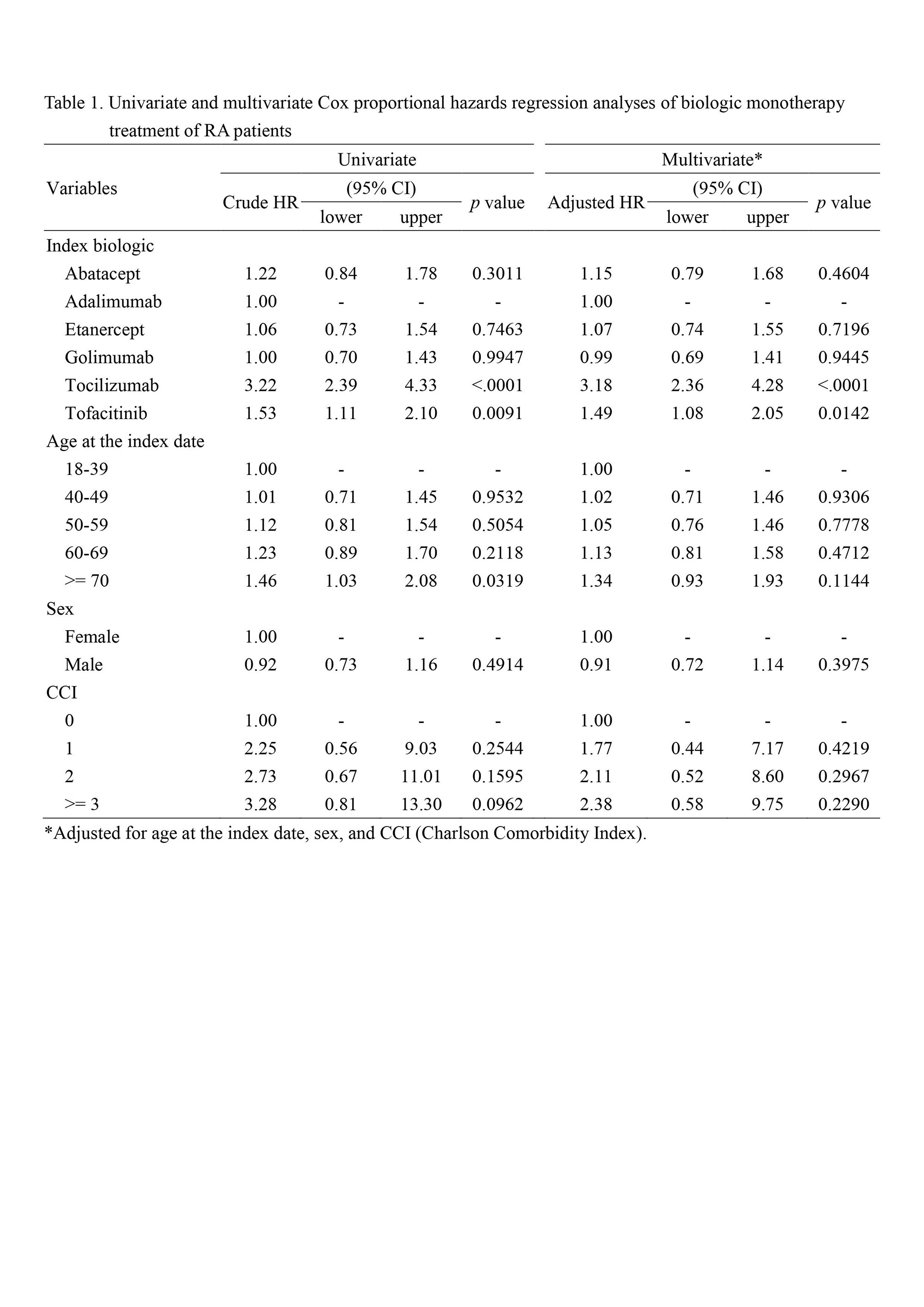
Results: The overall incidence rate of biologic monotherapy treatment of RA patients was 0.50 per 10 person-years, and the most prescribed medication was tocilizumab (the incident rate = 1.13), followed by tofacitinib (the incident rate = 0.55). Results of Cox proportional hazards regression analyses further asserted that tocilizumab was the most prescribed biologic monotherapy (adjusted hazard ratio = 3.18, 95% CI = 2.36-4.28, p < 0.0001) (Table 1).
Conclusion: There is a need for a monotherapy approach in the treatment of RA patients, and the arsenal of biologic monotherapy is incessantly growing. This nationwide population-based study has demonstrated that in the real world rheumatology practice of biologic monotherapy for RA patients, tocilizumab is the treatment of choice, whereas tofacitinib is the next viable option.
To cite this abstract in AMA style:
Li K, Huang K, Lin C, Chang C. Real World Rheumatology Practice of Biologic Monotherapy for the Treatment of Patients with Rheumatoid Arthritis: A Four-year Cohort Study Using a National Claims Database [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/real-world-rheumatology-practice-of-biologic-monotherapy-for-the-treatment-of-patients-with-rheumatoid-arthritis-a-four-year-cohort-study-using-a-national-claims-database/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-rheumatology-practice-of-biologic-monotherapy-for-the-treatment-of-patients-with-rheumatoid-arthritis-a-four-year-cohort-study-using-a-national-claims-database/