Session Information
Date: Sunday, October 26, 2025
Title: (0357–0386) Patient Outcomes, Preferences, & Attitudes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Over the last decade, Janus kinase inhibitors (JAKs) for RA have been increasingly used as a treatment option for patients who do not respond to biologic DMARD (bDMARD) treatments. The introduction of orally administered drugs, like JAKs, was expected to address poor therapy persistence rates associated with self-administered, injectable bDMARDs. The purpose of this analysis is to evaluate the persistence of JAKs used to treat RA in the United States using real-world commercial (Marketscan) and government (Medicare) insurance claims data.
Methods: We conducted a retrospective analysis of patients initiating their first JAK for RA using 2006-2021 Marketscan data (commercial health plan claims data) and 2010-2020 Medicare fee for service (FFS) data to assess 2-year JAK persistence. The last index date in the claims data that was allowable was 12/31/2019 for Medicare and 12/31/2020 forMarketscan, enabling the analysis to have the potential for at least 1 year of follow-up . Drug persistence was measured at 1 and 2 years using the following definition: continuous use of JAK without gap of >90 days (next prescription fill date is no later than previous prescription fill date + days of supply + 90) and did not switch to another JAK or bDMARD.
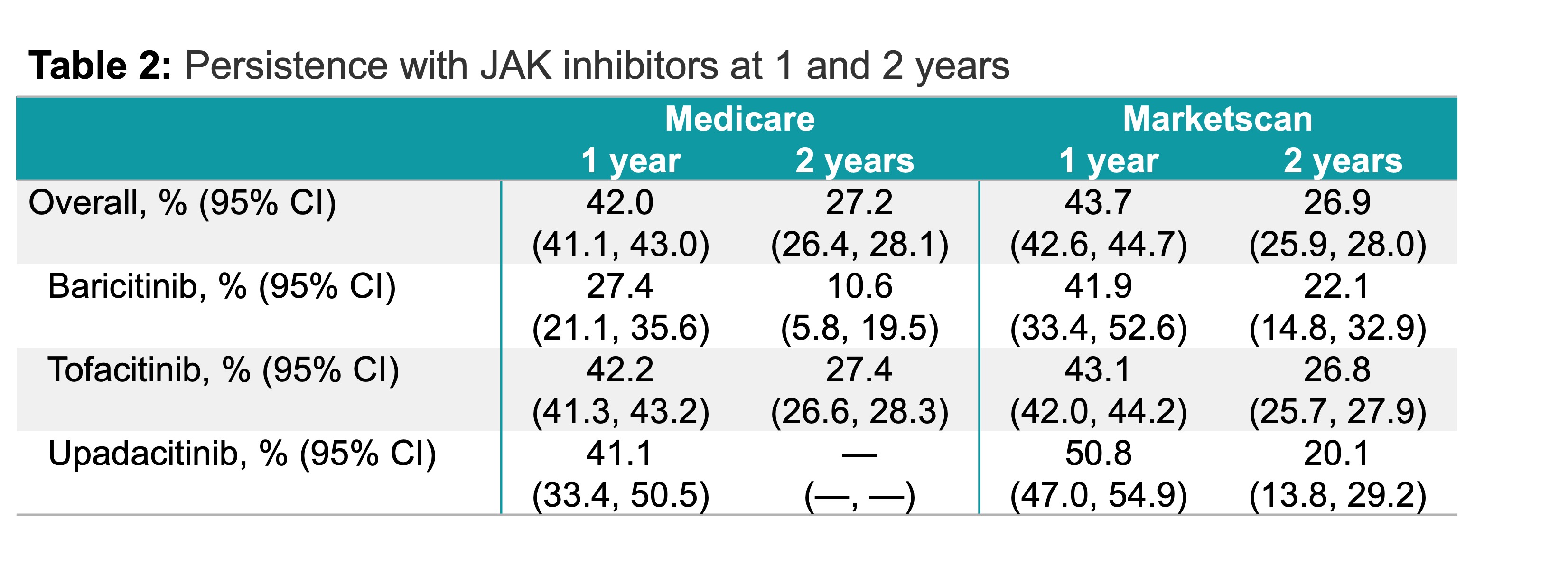
Results: In the Medicare cohort, there were 11,170 RA patients with an index date on or before 12/31/2019. Individuals had mean prior use of 2.1 ± 1.2 bDMARDs and 1.8 ± 1.0 cDMARDs. JAK persistence was 42.0% (95% CI: 41.1, 43.0) at 1 year and 27.2% (95% CI: 26.4, 28.1) at 2 years. Drug persistence differed across individual drugs with 1-year persistence of 27.4% (95% CI: 21.1, 35.6), 42.2% (95% CI: 41.3, 43.2), and 41.1% (95% CI: 33.4, 50.5) for baricitinib, tofacitinib and upadacitinib, respectively. At 2 years, 10.6% (95% CI: 5.8, 19.5) and 27.4% (95% CI: 26.6, 28.3) continued baricitinib and tofacitinib, respectively. There were no patients taking upadacitinib at 2 years, which was not unexpected given the end of the Medicare data (12/31/2020) and the timing of FDA approval of upadacitinib (2019). In the Marketscan cohort, there were 9,458 patients with an index date on or before 12/31/2020. Patients had mean prior use of 2.0 ± 1.1 bDMARDs and 1.7 ± 1.0 cDMARDs. Among these, 43.7% (95% CI: 42.6, 44.7) initiating a JAK were persistent at 1 year, and 26.9% (95% CI: 25.9, 28.0) were persistent at 2 years. At 1 year, 41.9% (95% CI: 33.4, 52.6), 43.1% (95% CI: 42.0, 44.2), and 50.8% (95% CI: 47.0, 54.9) of patients continued baricitinib, tofacitinib and upadacitinib, respectively. At 2 years, 22.1% (95% CI: 14.8, 32.9) of patients continued baricitinib, 26.8% (95% CI: 25.7, 27.9) continued tofacitinib, and 20.1% (95% CI: 13.8, 29.2) continued upadacitinib.
Conclusion: Persistence with initial JAK for RA in real-world settings was poor for all FDA-approved JAKs as measured in two independent data sources, and 2-year estimates were comparable between commercial and government health claims sources. Drug persistence differed across individual JAKs, with lower persistence rates for baricitinib compared to tofacitinib and upadacitinib. Compared to historical data, the introduction of oral JAKs does not appear to have improved therapy persistence, with < 30% treatment persistence at 2 years.
To cite this abstract in AMA style:
Curtis J, Holladay E, McCormick N, Su Y, Xie F, Dineen A. Real-World Persistence of Janus Kinase Inhibitors in Biologic-Experienced Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-persistence-of-janus-kinase-inhibitors-in-biologic-experienced-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-persistence-of-janus-kinase-inhibitors-in-biologic-experienced-patients-with-rheumatoid-arthritis/

.jpg)
.jpg)