Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The use of Janus Kinase inhibitors (JAKi) to treat RA and PsA in a real-world setting has not been well described. Our aim was to investigate the place in treatment pathway, retention and factors associated with discontinuation of JAKi prescribed for RA and PsA in routine care.
Methods: A retrospective review of all patients treated with Baricitinib (B), Filgotinib (F), Tofacitinib (T) and Upadacitinib (U) in 6 centres was conducted from time of first use of any of these agents in routine care until November 2024. Standardized data was collected including demographics, place in treatment pathway, concurrent MTX and CS use, duration of treatment and reason for discontinuation. Retention rate was calculated using Kaplan-Meier analysis. Factors associated with JAKi discontinuation were assessed by multivariable Cox regression analyses.
Results: The case notes of 985 patients were reviewed, 79.5% female, mean age 60 (range 19 – 89) years. The JAKi was B in 658 (67%), F in 127 (13%), T in 100 (10%) and U in 100 (10%). The disease indications were RA in 849 (86%; B 648, F 127, T 47, U 27), PsA in 136 (14%; B 10, F 0, T 53, U 73). MTX was co-prescribed in 43%, subsequently discontinued in 6%. CS was co-prescribed in 34%, subsequently tapered in 18% and discontinued in 62%.For all cases, JAKi were prescribed 1st line before biologic DMARD in 7.5%, 2nd line after bDMARD 13.7%, 3rd line 29% and 4th line or later in 49.8%. Median retention for all JAKi was 49 months (range 1-85, 95% C.I. 42.62-55.37), and 52.8% remained on treatment at time of analysis, with no difference between RA and PsA populations. The median retention on B was 52 months and T 24 months (p < 0.0011), with no difference in retention between other pairwise JAKi comparisons. See Figures 1 and 2.Multivariable regression analyses showed a significantly higher relative risk of JAKi discontinuation for older age, female sex and treatment with Tofa compared to Bari. For RA patients co-prescription with MTX had a significantly lower risk of JAKi discontinuation, Table 1.JAKi were discontinued in 46.4%; primary inefficacy 9.8%, secondary inefficacy 11.8%, adverse events 18.5% and 6.3% pre-emptively due to the Oral Surveillance study findings. There were no significant differences in reasons for discontinuation between RA and PsA, nor individual JAKi. Venous thromboembolic events (VTE) occurred in 1.4%, event rate 0.57 / 100 patient years; 10 pulmonary embolism (PE) and 4 deep vein thrombosis (DVT), with mean time to event 29 months, range 6 – 75 months. Other adverse events included cerebrovascular events in 1.2%, 0.49 / 100 patient years, cardiac events in 0.7%, 0.29 / 100 patient years and cancer in 0.9%, 0.37 / 100 patient years.
Conclusion: JAKi are generally used late in the DMARD treatment pathway in routine care, with high retention rates and a CS sparing effect was evident. Significantly higher risks for discontinuation were female sex, older age, Bari versus Tofa treatment, and in RA patients use without MTX. The place in treatment pathway conferred no effect on retention. Adverse events were in line with this class of immune suppressive therapies including VTE, where PE outnumber DVT events.
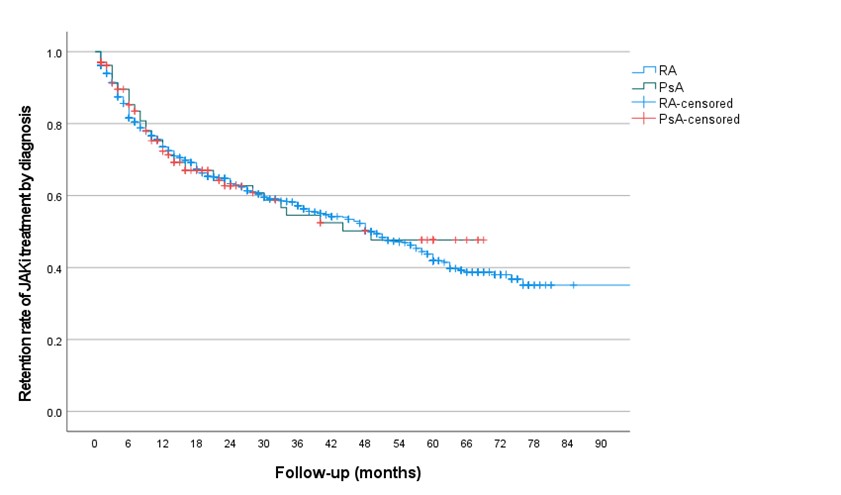 Figure 1. Kaplan Maier retention curve for patients with RA and PsA treated with any JAK inhibitor
Figure 1. Kaplan Maier retention curve for patients with RA and PsA treated with any JAK inhibitor
.jpg) Figure 2. Kaplan Maier retention curve for all patients treated with individual JAK inhibitors
Figure 2. Kaplan Maier retention curve for all patients treated with individual JAK inhibitors
.jpg) Table. Multivariable regression analysis showing relative risk for JAKi discontinuation; all patients and RA and PsA patients
Table. Multivariable regression analysis showing relative risk for JAKi discontinuation; all patients and RA and PsA patients
To cite this abstract in AMA style:
Nathan J, Farisogullari B, Jones N, Ayoub M, Brown J, Levy S, Brader J, Ersoy A, Israni B, Lloyd M, Latheef A, Hill D, Taylor A, Arachchige D, Chitsu H, Linklater H, Lather K, Gompels L, Elfar E, Malaiya R, Machado P, Kiely P. Real-World Experience of Janus Kinase Inhibitors; Retention and Factors Associated with Discontinuation [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-experience-of-janus-kinase-inhibitors-retention-and-factors-associated-with-discontinuation/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-experience-of-janus-kinase-inhibitors-retention-and-factors-associated-with-discontinuation/
