Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with rheumatic diseases (RD) are at increased risk for herpes zoster (HZ) due to disease -related aberrant immune response, comorbid conditions and anti-rheumatic treatments. There are limited real life data regarding the safety of the recently available adjuvanted Recombinant Zoster Vaccine (RZV) in patients with RD. This prospective study aimed to assess RZV safety as well as the rate of post-vaccination disease flares in RD patients.
Methods: Prospective, multicenter, non-interventional study involving patients with RD having an indication for RZV vaccination (GReaZE study). Patients entering the trial were prospectively monitored for a period of 3 months after the initial RZV vaccination. Data on demographics, clinical and disease characteristics, current and previous treatments were collected at baseline (1st RZV dose), 1 month (2nd RZV dose) and 3 months later. All patients received diaries where they were asked to record pre-defined adverse events (AEs) and their severity for seven days following vaccination with each RZV dose. Disease flares were defined as a clinically significant increase in disease activity measured by indices specific to relevant RD or use of corticosteroids to control an exacerbation of the underlying disease within the period of monitoring.
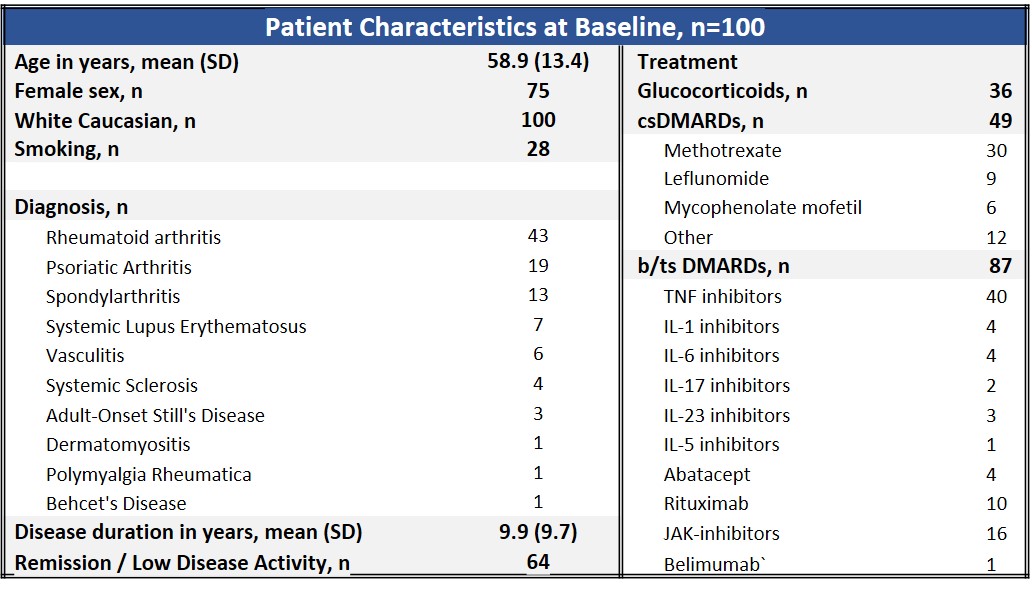
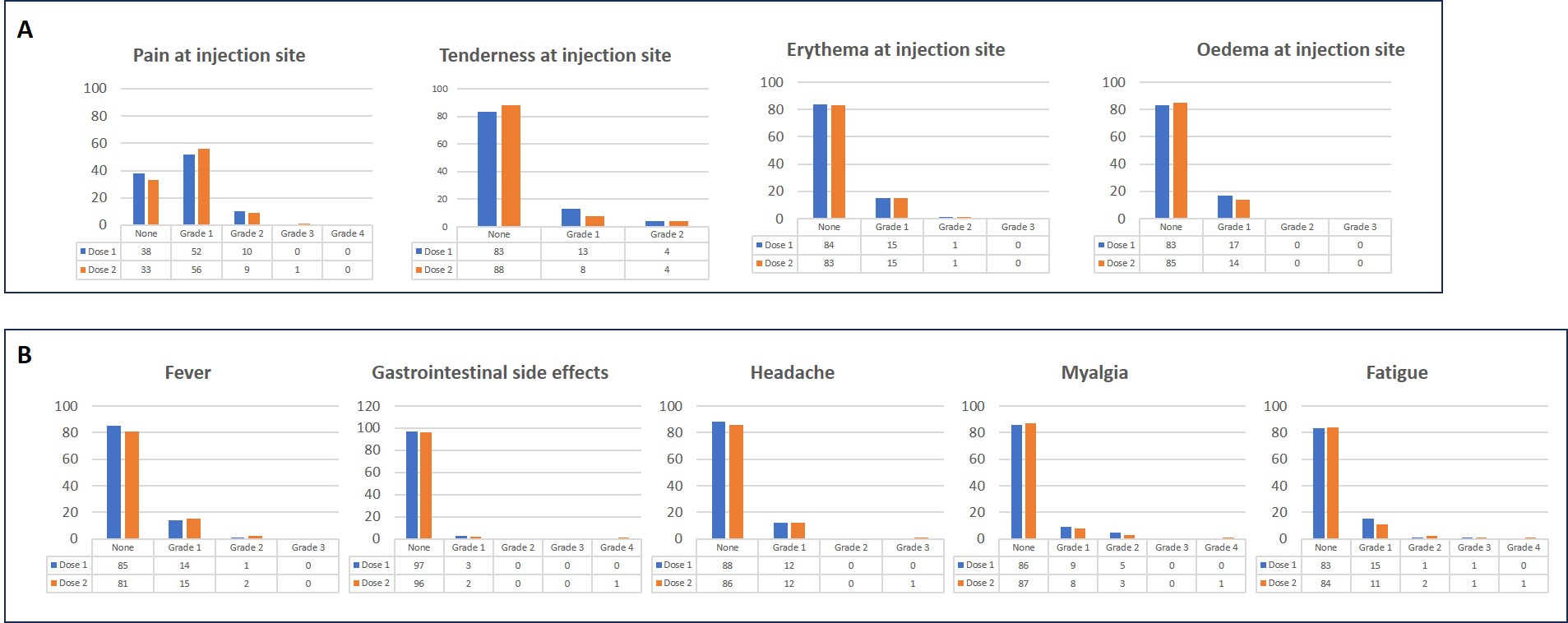
Results: Data from the first one hundred patients entering the GReaZE study are presented (table 1). Patients were predominantly female (75%), non-smokers (72%) with a mean (SD) age of 58.9 (13.4) years and mean (SD) disease duration of 9.9 (9.7) years. The most common RD diagnoses were rheumatoid arthritis (43%), psoriatic arthritis (19%) and axial spondylarthritis (15%). The majority of patients were in remission/low disease activity before RZV vaccination (64%), while being on biologic or targeted synthetic DMARDs (87%) and conventional DMARDs (49%). Seven patients (7%) had a history of previous herpes zoster infection. All patients, except for one who developed herpes zoster after the first dose, received both doses of the vaccine. The most common localized AE was pain at the injection site (62% and 66% after the 1st and 2nd dose respectively), while the most frequent systemic AE was fever (14% and 18% after the 1st and 2nd dose respectively). The majority of AEs were mild (figure 1) and of short duration (median (IQR): 3 (2-4.5) days) with the exception of one patient who was hospitalized after the second RZV dose due to systemic side effects (fever, headache, diarrhea and vomiting). The development of AEs was more frequent in women (62% vs 13%, p=0.006), but did not differ with age, diagnosis, type of DMARD treatment, glucocorticoids, disease duration or HZ history. Only 2 patients experienced a mild disease flare during the follow up period. Both presented after the second RZV dose and necessitated short treatment with glucocorticoids.
Conclusion: RZV was in general well tolerated in patients with RD with the most common side effects being localized and short-lived. The post-RZV disease flare rate in our prospective real-world cohort was low (2%) and flares were mild and easily manageable. This data could inform discussions with RD patients on RZV vaccination.
To cite this abstract in AMA style:
Koutsianas C, Apanomeritaki A, Mavrea E, Dimopoulou N, Nitsa M, TSALAPAKI C, Giannakopoulou I, Vassilopoulos D. Real World Evidence on the Safety of the Adjuvanted Recombinant Zoster Vaccine in Patients with Rheumatic Diseases – Preliminary Results from a Prospective Multicenter Observational Greek Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/real-world-evidence-on-the-safety-of-the-adjuvanted-recombinant-zoster-vaccine-in-patients-with-rheumatic-diseases-preliminary-results-from-a-prospective-multicenter-observational-greek-stud/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-evidence-on-the-safety-of-the-adjuvanted-recombinant-zoster-vaccine-in-patients-with-rheumatic-diseases-preliminary-results-from-a-prospective-multicenter-observational-greek-stud/