Session Information
Date: Monday, November 18, 2024
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Interstitial lung disease (ILD) is a serious complication of rheumatoid arthritis (RA), with a prevalence that ranges from 7 to 30% patients with RA, including subclinical ILD. Treatment of RA-ILD remains a challenge. The inflammatory activity of the disease should be managed with disease-modifying antirheumatic drugs (DMARDs). Nonetheless, approximately one third of these patients may develop a progressive ILD phenotype (with worsening respiratory symptoms, decline of lung function and/or increased fibrosis extent), despite the use of immunosuppressants. Antifibrotics could be a complementary therapy in these subjects. In this sense, a subgroup analysis of patients with RA-ILD from the INBUILD trial has recently been published, showing favorable results with nintedanib (NINTE). However, real-world data on the effectiveness of NINTE in RA-ILD patients with a progressive phenotype in clinical practice is scarce. The aim of this study was to assess this issue.
Methods: National multicenter study of RA-ILD patients to whom NINTE was added due to progressive fibrosing ILD. Demographic and clinical variables were collected from all patients and compared with those of RA-ILD patients included in the INBUILD trial (n=89, 42 treated with NINTE and 47 with placebo). Forced vital capacity (FVC) evolution was the primary endpoint. Results were expressed as mean ± SD or median [25th – 75th IQR] for normally or non-normally distributed variables, respectively. For the comparison of mean absolute change of FVC between two time points, paired Student’s t-test was used.
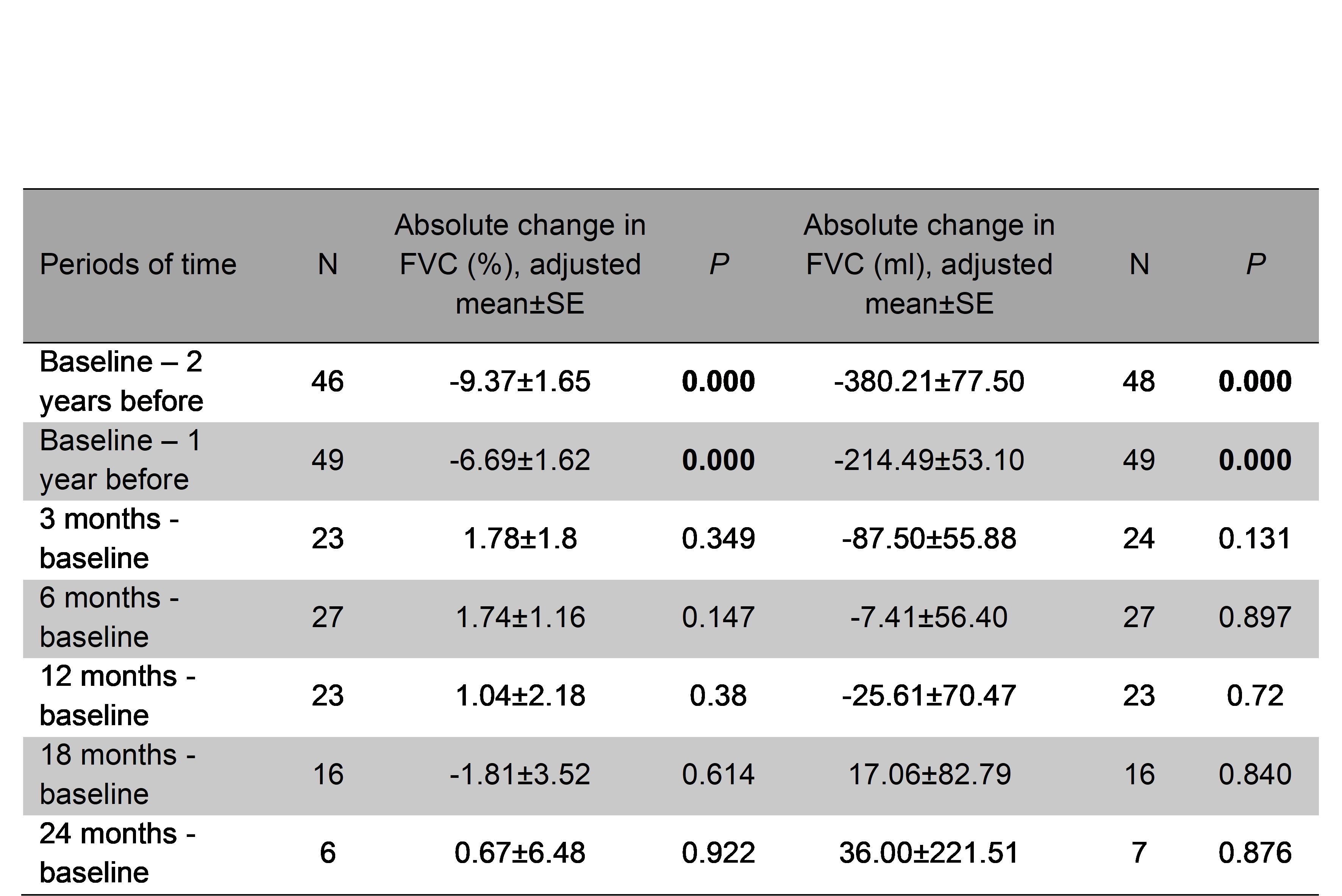
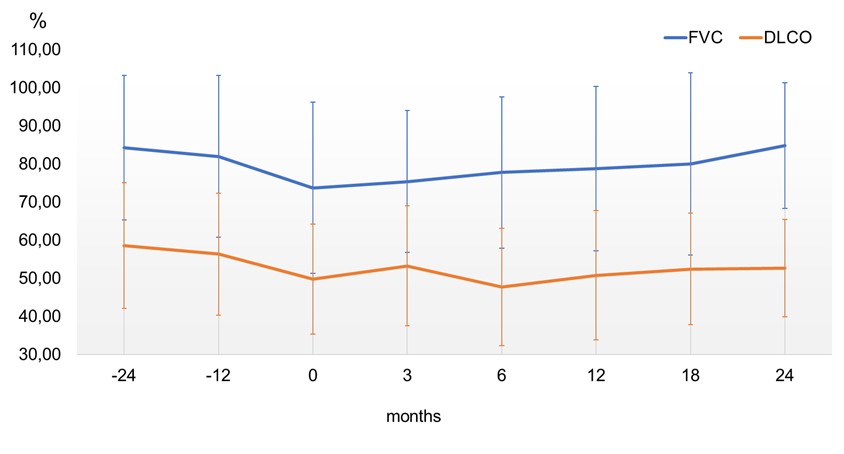
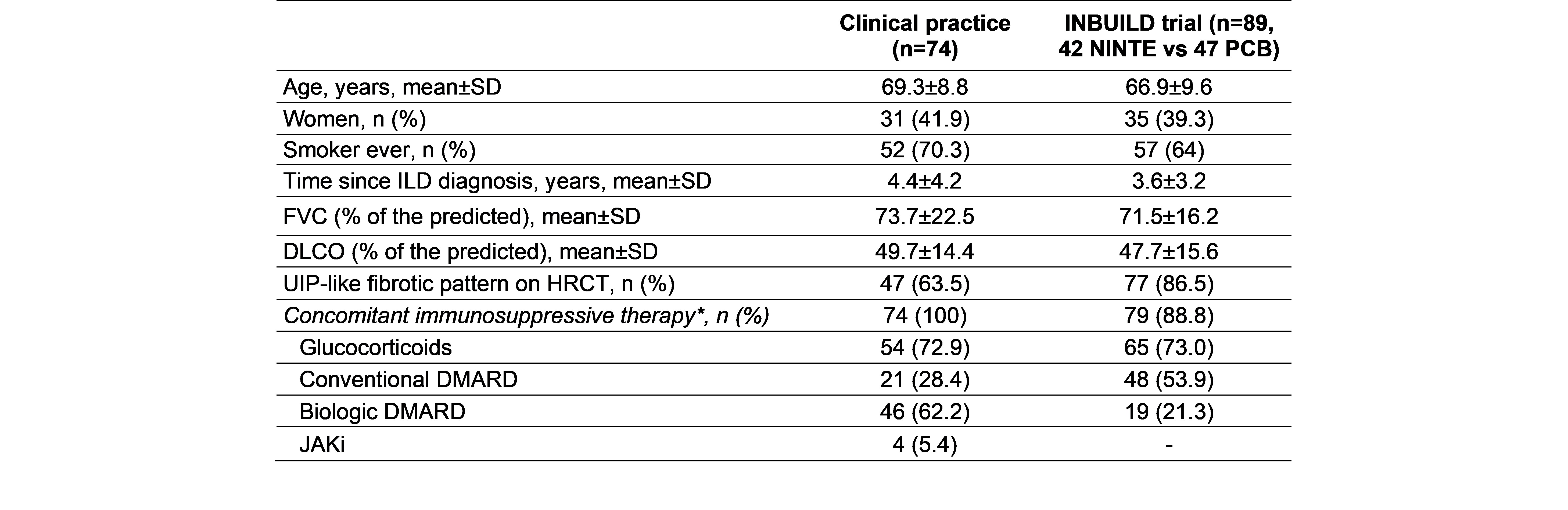
Results: A total of 74 patients (31 women/43 men) were collected, mean age of 69.3±8.8 years. Median ILD duration up to antifibrotic initiation was 51 [22-77.5] months. NINTE was administered in combination with corticosteroids (n=54), cDMARD (n=21), bDMARD (n=46) and/or JAKi (n=4). Mean FVC one year before NINTE start was 81.9±21.2 (% pred.), whilst mean baseline FVC was 73.7±22.5 (% pred.). After a median follow-up of 15 [4-23] months, no significant decline in mean FVC or DLCO values was observed (Figure 1). In the intra-individual analysis (Table 1), the absolute change in FVC at 12 months from baseline was of 1.04±2.18 % and -25.61±70.47 ml. In addition, the absolute change in FVC (ml) at 18 and 24 months from baseline turned into positive (17.06±82.79 and 36.00±221.51 ml, respectively). Gastrointestinal adverse events were the most common reason for NINTE discontinuation. The retention rate was 78.4% (58 patients) at the end of follow-up. In contrast with the INBUILD trial, RA-ILD patients from clinical practice were older, had a higher tobacco exposure, time since ILD diagnosis was longer and treatment with combined immunosuppressants was more frequent (Table 2). However, baseline mean values of FVC and DLCO were similar in both groups.
Conclusion: NINTE seems to slow ILD progression in patients with RA-ILD. In clinical practice, patients are treated later in the evolution of the disease, but results are satisfactory. Combination of NINTE and DMARDs in RA-ILD is possible and safe.
*Patients from de INBUILD trial were restricted to use at baseline: glucocorticoids if >20 mg/day prednisone or equivalent, mycophenolate mofetil, cyclophosphamide, cyclosporine, tacrolimus, azathioprine and rituximab.
ACPA, anti-citrullinated protein antibodies; DLCO, diffusing capacity of the lung for carbon monoxide; DMARD, disease-modifying antirheumatic drug; FVC, forced vital capacity; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; IQR, interquartile range; JAKi, JAK inhibitor; mMRC: modified Medical Research Council scale; NINTE, nintedanib; PCB, placebo; RA, rheumatoid arthritis; RF, rheumatoid factor; SD, standard deviation; UIP, usual interstitial pneumonia.
To cite this abstract in AMA style:
Atienza-Mateo B, Serrano-Combarro A, Loarce J, Vegas Revenga N, Martín López M, Castañeda S, Melero-Gonzalez R, Mena Vázquez N, Carrasco-Cubero C, Díez Morrondo C, Castro-Corredor D, Vázquez Rodríguez T, García-Valle A, Bonilla G, Rodriguez M, Brana Abascal I, Rojas Herrera S, Sarmiento-Monroy J, Andújar-Brazal P, Ferrer D, Blanco-Alonso R. Real-World Evidence of the Antifibrotic Nintedanib in Rheumatoid Arthritis-Interstitial Lung Disease. National Multicenter Study of 74 Patients from Clinical Practice [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/real-world-evidence-of-the-antifibrotic-nintedanib-in-rheumatoid-arthritis-interstitial-lung-disease-national-multicenter-study-of-74-patients-from-clinical-practice/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-evidence-of-the-antifibrotic-nintedanib-in-rheumatoid-arthritis-interstitial-lung-disease-national-multicenter-study-of-74-patients-from-clinical-practice/