Session Information
Date: Monday, November 11, 2019
Title: RA – Diagnosis, Manifestations, & Outcomes Poster II: Treatments, Outcomes, & Measures
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Infections are common safety events monitored in RA patients.1 ACR guidelines limit the use of live vaccines in patients who are on biologic (b)DMARDs or Janus kinase inhibitor (JAKi).2 The presence of comorbidities and treatment-related serious infections may lead to treatment discontinuation. This study aimed to update estimates of the prevalence and incidence of various infections among patients switching from a first bDMARD to another treatment and estimate healthcare costs associated with them.
Methods: In a US health plan claims database, we selected adult RA (≥2 RA diagnoses) patients who newly initiated a bDMARD (1/1/2012–3/31/2017) and switched to another bDMARD or JAKi (index date; ID). All patients had continuous enrollment 12-months pre- and ≥12-months post-ID. Prevalence (12-month pre-ID) and while-on-treatment incidence per 100 patient-years (P100PY) of serious infections, opportunistic infections, and herpes zoster were evaluated overall and by index treatment. For incidence rates, patients with no prior events were followed from ID until the first event or the end of index treatment. Unadjusted mean annualized all-cause healthcare costs (total, medical, and pharmacy) were assessed during index treatment and compared between patients with and without infections of interest.
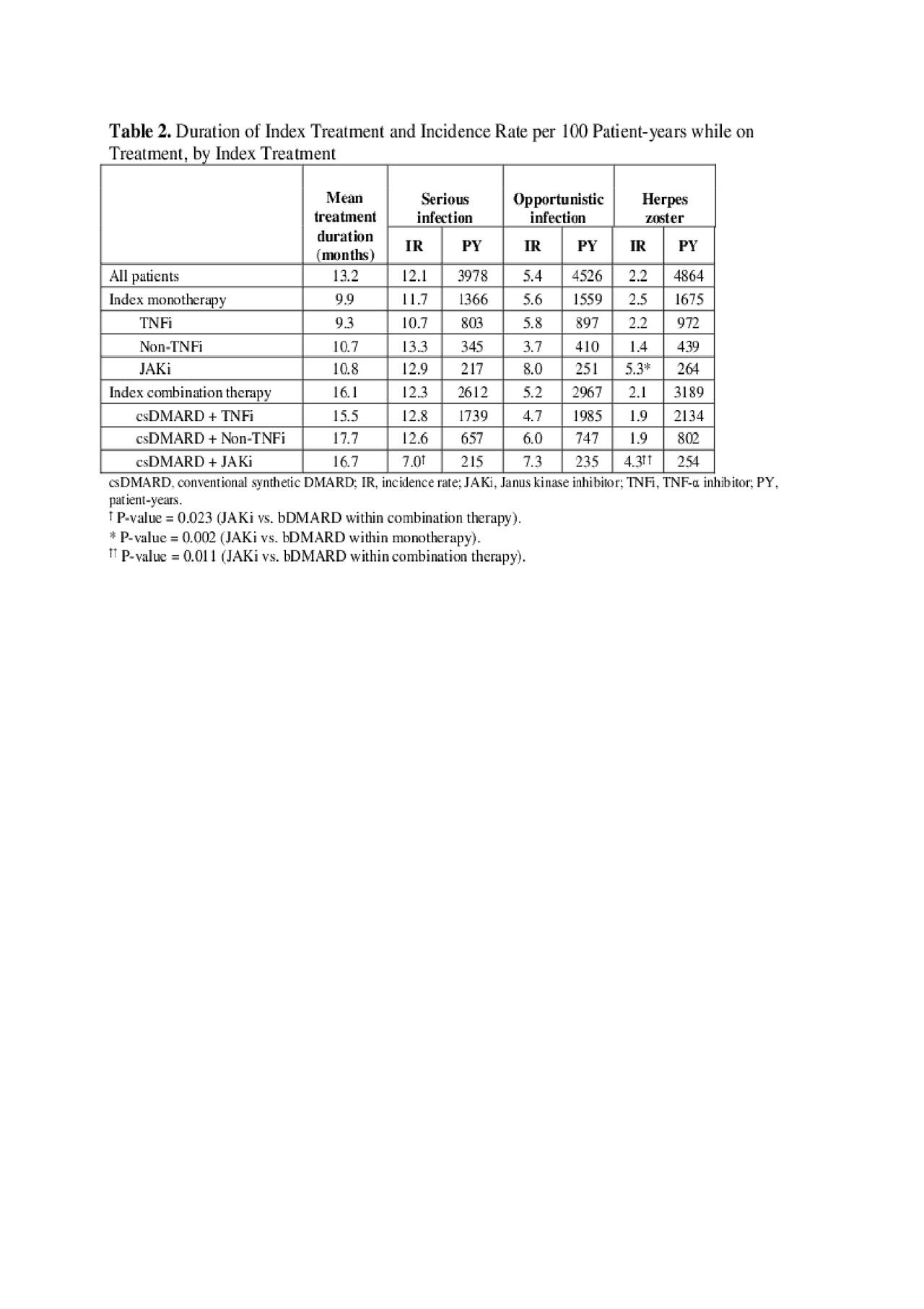
Results: 4,656 patients switched from a first bDMARD to another treatment: median age 54 years, 78% female, mean duration of RA 1.8 years. After initial bDMARD, 46% of patients used monotherapy (61% TNF-α inhibitor (TNFi), 24% non-TNFi, 15% JAKi), and 54% used another bDMARD or JAKi in combination with csDMARD (69% TNFi + csDMARD, 23% non-TNFi + csDMARD, 8% JAKi + csDMARD). Pre-index prevalence rates ranged 12–17% for serious infections, 5–7% for opportunistic infections, and 2–3% for herpes zoster and were comparable among treatments (Table 1). While on index treatment, incidence rates ranged 7–13 P100PY for serious infections, 4–8 P100PY for opportunistic infections, and 1–5 P100PY for herpes zoster. Incidence rates varied among different treatments (Table 2). Total unadjusted healthcare costs were significantly higher in patients with serious infections ($66,588 vs $53,263; p< 0.0001) or opportunistic infections ($59,525 vs $54,369; p=0.023) than without these infections. Cost differences were primarily associated with medical costs (Table 3).
Conclusion: Serious infections, opportunistic infections, and herpes zoster affected RA patients switching from their first bDMARD to another treatment. While on index treatment, incident infections were associated with increased unadjusted costs, posing additional economic burden. Adjusted analyses are needed to estimate the economic burden of these infections accounting for difference in patient cohorts. Clinicians should factor the burden of infections into their clinical decisions.
Reference
- Ramiro S, et al. Ann Rheum Dis. 2017; 76: 1101-1136.
- Singh JA, et al. Arthritis & Rheumatology. 2016; 68:1-26.
To cite this abstract in AMA style:
Dore R, Antonova J, Huang H, Chang L, Wang X, Genovese M. Real-World Evidence: Infections Among RA Patients Switching from First Biologic DMARD to Another Treatment in the US [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/real-world-evidence-infections-among-ra-patients-switching-from-first-biologic-dmard-to-another-treatment-in-the-us/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-evidence-infections-among-ra-patients-switching-from-first-biologic-dmard-to-another-treatment-in-the-us/