Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Methotrexate (MTX) is a foundational agent in the management of rheumatoid arthritis (RA). While oral MTX is widely prescribed, its subcutaneous (SC) formulation may enhance bioavailability and reduce gastrointestinal intolerance. Despite these theoretical advantages, real-world comparative data—particularly incorporating musculoskeletal ultrasound (MSUS)—remain sparse. This study aimed to compare clinical efficacy, sonographic synovial response, and safety between SC and oral MTX, adjusting for potential confounders such as MTX dosage and baseline disease activity.
Methods: In this retrospective observational study, we analyzed 96 RA patients from two Japanese centers who initiated either SC MTX (n = 75) or oral MTX (n = 21) between April 2022 and March 2025. All patients fulfilled the 2010 ACR/EULAR criteria. Disease activity indices—including TJC28, SJC28, DAS28-CRP, SDAI, and CDAI—were assessed at baseline and at 1, 3, and 6 months. Serial MSUS assessments were performed in the SC group to evaluate synovitis using power Doppler (PD) and gray scale (GS) imaging (semi-quantitative scale 0–3). Multivariable linear regression models were applied to adjust for baseline scores and MTX starting doses.
Results: At 6 months, SC MTX was associated with significantly greater reductions in TJC28 (–4.0 vs. –1.0; p = 0.0229), DAS28-CRP (–1.28 vs. –0.57; p = 0.0117), SDAI (–6.8 vs. –2.0; p = 0.0005), and CDAI (–7.3 vs. –2.8; p = 0.0414). SJC28 changes did not differ significantly (p = 0.8711). Multivariable analysis adjusting for baseline MTX dose confirmed the superiority of SC MTX in improving DAS28-CRP (β = –0.74, p = 0.005), CDAI (β = –7.15, p < 0.001), SDAI (β = –7.15, p < 0.001), and TJC28 (β = –2.34, p = 0.036), while no significant difference was observed for SJC28 (β = –0.61, p = 0.438). PD and GS scores declined steadily in the SC group, although PD signals recurred in some patients at month 6. No serious adverse events were observed. Mild hepatic and renal function changes occurred more frequently in the oral MTX group, though not significantly.
Conclusion: Subcutaneous MTX demonstrated enhanced clinical and ultrasonographic outcomes compared to oral MTX, independent of baseline disease activity or starting dose. These findings support broader adoption of SC MTX as a practical and effective strategy for RA management prior to escalation to biologic agents. The inclusion of imaging and dose-adjusted outcomes strengthens the generalizability and clinical relevance of this study.
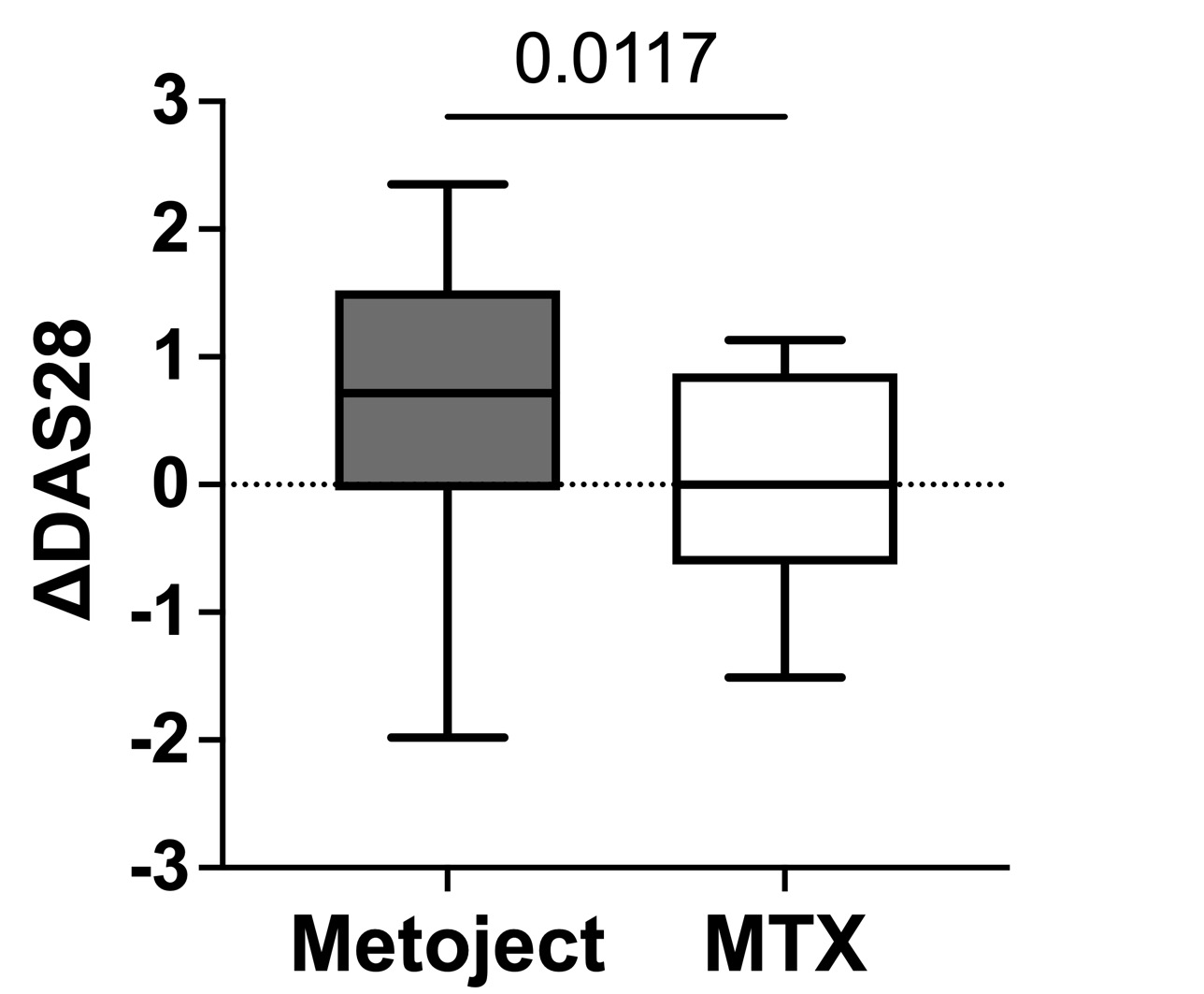 Table 1. Baseline Demographic and Clinical Characteristics
Table 1. Baseline Demographic and Clinical Characteristics
.jpg) Figure 2. Longitudinal changes in disease activity indices
Figure 2. Longitudinal changes in disease activity indices
DAS28-CRP scores at baseline, 1, 3, and 6 months in the subcutaneous and oral MTX groups.
.jpg) Figure 3. Ultrasound findings in the subcutaneous MTX group
Figure 3. Ultrasound findings in the subcutaneous MTX group
Representative musculoskeletal ultrasound (MSUS) images demonstrating resolution (upper panel) and recurrence (lower panel) of power Doppler (PD) signal at baseline, 3 months, and 6 months.
To cite this abstract in AMA style:
Komagamine M, Taguchi S, Komagamine M, Naka T, Fujimoto M. Real-World Evidence for the Superiority of Subcutaneous Methotrexate in RA: A Comparative Observational Study with Ultrasonographic Assessment [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-evidence-for-the-superiority-of-subcutaneous-methotrexate-in-ra-a-comparative-observational-study-with-ultrasonographic-assessment/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-evidence-for-the-superiority-of-subcutaneous-methotrexate-in-ra-a-comparative-observational-study-with-ultrasonographic-assessment/
