Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
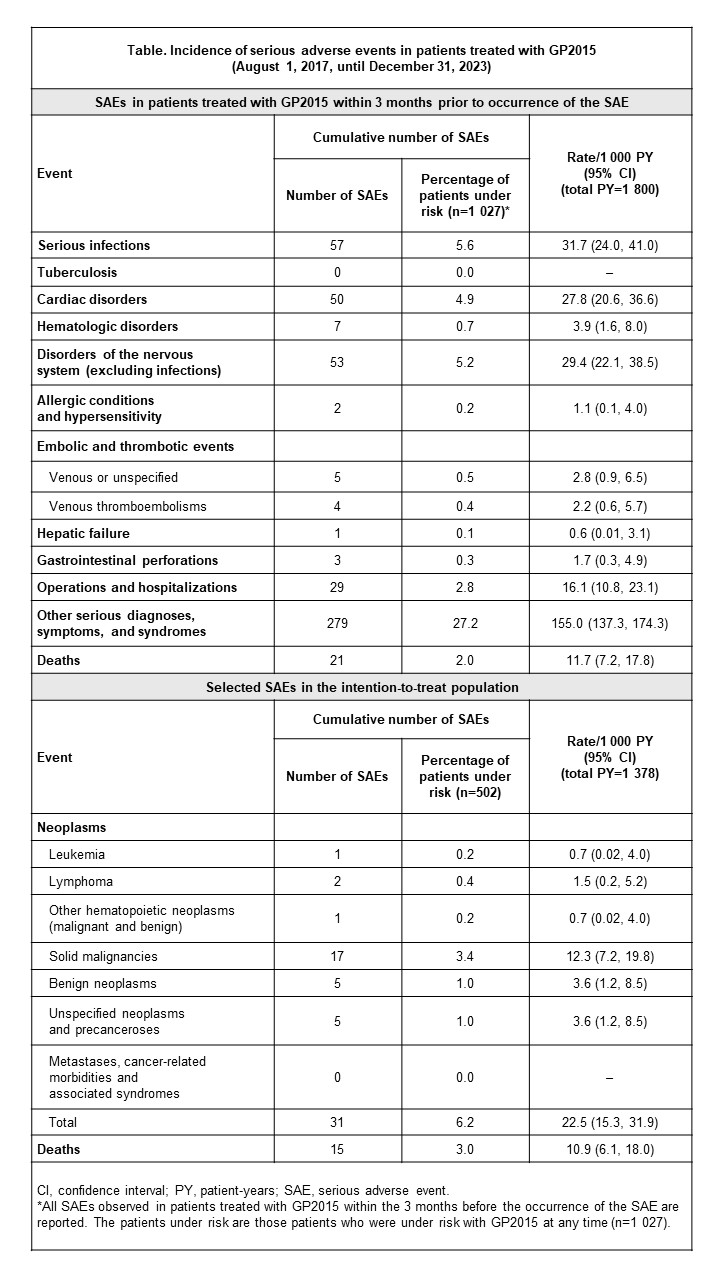
Background/Purpose: GP2015 is an etanercept biosimilar indicated for the treatment of rheumatoid arthritis (RA).1 The objective of this abstract is to describe the baseline characteristics and safety outcomes of patients with RA treated with GP2015 enrolled in the Rheumatoide Arthritis: Beobachtung der Biologika-Therapie (RABBIT) registry. The registry is a prospective observational cohort study that aims to assess the long-term safety of biologic agents in patients with RA.2
Methods: Adult patients with RA who were treated with GP2015 were included. Analysis of this cohort from RABBIT was based on the use of routinely provided safety data from standardized reports to the Marketing Authorization Holder and included adult patients with RA who initiated GP2015 during observation in the registry. The analysis included patients who initiated GP2015 treatment from August 1, 2017 onwards until the data cut-off date on December 31, 2023. Descriptive analyses were performed to characterize the data. Cumulative incidence of serious adverse events (SAEs) was analyzed as incidence per patient-years.
Results: Of patients enrolled in the registry, 502 patients initiated GP2015 at enrollment, 525 initiated GP2015 during observation, and 68.3% were female (n=343). Mean age was 60.8±12.9 years and mean duration from symptom onset was 8.0±8.4 years; 13.1% of patients were previously treated with biologic/targeted synthetic disease-modifying antirheumatic drugs and 45% had ≥3 comorbidities. Mean Disease Activity Score-28 was 4.6±1.2 and mean pain Visual Analog Scale score was 5.5±2.2. Incidences of SAEs in all patients treated with GP2015 (n=1 027) are shown in the Table. After approximately 1.7 years’ follow-up, the most common SAEs were infections (n=57), cardiac disorders (including coronary artery disease, heart failure, and myocardial infarction) (n=50), and disorders of the nervous system (including stroke and other disorders of the central or peripheral nervous system) (n=53).
Conclusion: Patient characteristics were generally balanced with the profile of patients with RA from a previous study,3 and no new safety signals were identified. Overall, the real-world incidence of SAEs in patients treated with GP2015 was low and comparable with the safety profile of reference etanercept.4
References
1. European Medicines Agency (EMA). Erelzi Summary of Product Characteristics
2. Richter A, et al. Clin Exp Rheumatol 2016;34:S79–86
3. Chadwick L, et al. Curr Rheumatol Rep 2019;20:84
4. Feist E, et al. Rheumatol Ther 2022;9:621–35
To cite this abstract in AMA style:
Baraliakos X, Salvati C, Bachinskaya E, Sinha S, Krüger K. Real-World Evidence for GP2015 in Patients with Rheumatoid Arthritis: Safety Outcomes from German Observational Data [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/real-world-evidence-for-gp2015-in-patients-with-rheumatoid-arthritis-safety-outcomes-from-german-observational-data/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-evidence-for-gp2015-in-patients-with-rheumatoid-arthritis-safety-outcomes-from-german-observational-data/