Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: RA patients have an increased risk of malignancy1 and deep-vein thrombosis and pulmonary embolism (DVT/PE)2 and high prevalence of anemia.3 The risks of anemia and malignancy vary by prior DMARD exposure.1 Comorbidities and treatment side effects may lead to treatment discontinuation and increase healthcare costs. Existing estimates of anemia, DVT, PE, and malignancy are outdated or not specific to patients initiating next treatment after first biologic (b)DMARD. This study aimed to estimate prevalence, incidence, and associated costs of these conditions in RA patients switching from a first bDMARD to another treatment.
Methods: In a US health-plan claims database, we selected adult RA patients (≥2 RA diagnoses ≥30 days apart) who initiated their first bDMARD (1/1/2012–3/31/2017) and switched to another bDMARD or Janus kinase inhibitor (JAKi; index date, ID). Patients had continuous health plan enrollment for 12 months pre-ID and ≥12 months post-ID. The prevalence and incidence of anemia, DVT, PE, DVT or PE, and malignancy (excluding nonmelanoma skin cancer) were assessed by index therapy. Incidence counted patients with new events per 100 patient-years (P100PY) while on treatment. We compared mean per-patient-per-year (PPPY) costs (total, medical, and pharmacy) in patients with and without these conditions.
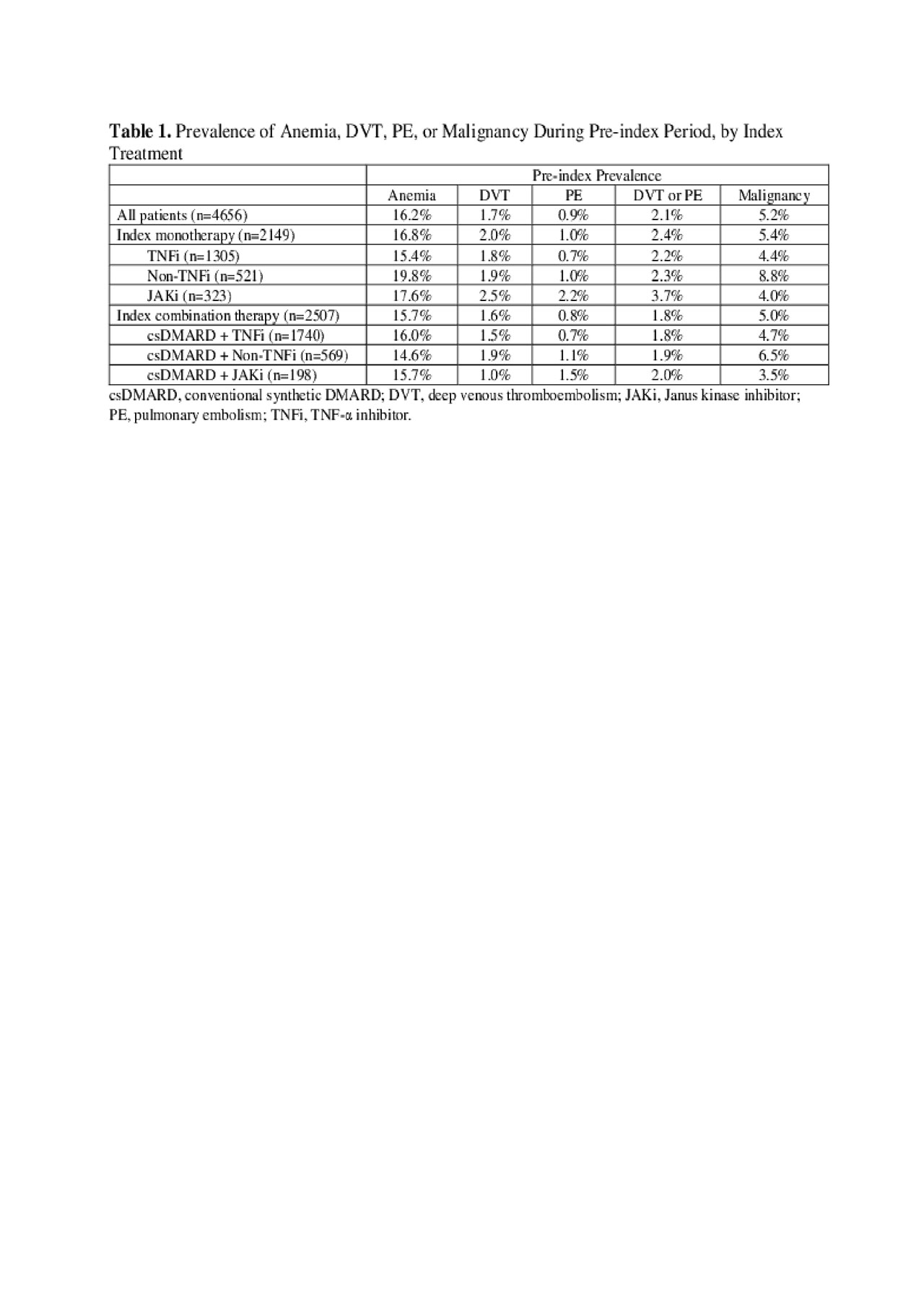
Results: In 4656 patients who switched from first bDMARD (median age 54 years, 78% female), median RA duration was 1.5 years. Upon discontinuation of the first bDMARD, 46% of patients received a monotherapy (61% TNF-α inhibitor (TNFi), 24% non-TNFi, 15% JAKi), and 54% received a conventional synthetic (cs)DMARD-combination therapy (69% TNFi + csDMARD, 23% non-TNFi + csDMARD, 8% JAKi + csDMARD). The 12-month pre-ID prevalence ranged 14.6–19.8% for anemia, 1.0–2.5% for DVT, 0.7–2.2% for PE, 1.8–3.7% for DVT or PE, and 3.5–8.8% for malignancy (Table 1). Overall, P100PY incidence rates were 6.9 for anemia, 0.7 for DVT, 0.3 for PE, 0.9 for DVT or PE, and 2.0 for malignancy. Incidence rates varied among different treatment classes (Table 2). Total PPPY unadjusted healthcare costs were higher in RA patients with vs without condition: anemia $66,896 vs $53,853; DVT $78,461 vs $54,462; PE $99,321 vs $54,483; DVT/PE $88,610 vs $54,305; malignancy $76,741 vs $54,172, all p< 0.001. Cost differences were primarily associated with medical costs (Table 3).
Conclusion: Anemia, DVT, PE, and malignancies affected patients who switched from first bDMARD to another treatment; new cases occurred and varied by treatment received. These conditions were associated with increased unadjusted healthcare costs, primarily driven by medical costs. Future studies are needed to adjust costs by accounting for differences in patient cohorts. The prevalence of these conditions (and the risk of their development as treatment side effects) should be factored into the selection of most optimal treatment.
References
- Wilton KM, et al. Rheumatol Ther 2017;4:333-47.
- Ungprasert P, et al. Clin Rheumatol 2014;33:297-304.
- Wilson A, et al. Am J Med 2004;116 Suppl 7A:50S-7S.
To cite this abstract in AMA style:
Dore R, Antonova J, Gorritz M, Chang L, He J, Genovese M. Real-World Evidence: Clinical and Economic Burden of Anemia, Venous Thromboembolism, and Malignancy Among Rheumatoid Arthritis Patients Switching from First Biologic DMARD to Another Treatment in the US [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/real-world-evidence-clinical-and-economic-burden-of-anemia-venous-thromboembolism-and-malignancy-among-rheumatoid-arthritis-patients-switching-from-first-biologic-dmard-to-another-treatment-in-the/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-evidence-clinical-and-economic-burden-of-anemia-venous-thromboembolism-and-malignancy-among-rheumatoid-arthritis-patients-switching-from-first-biologic-dmard-to-another-treatment-in-the/