Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The efficacy and safety of upadacitinib (UPA), an oral reversible Janus kinase inhibitor, have been shown in patients (pts) with moderate/severe RA in the SELECT clinical trial program, both as monotherapy and as part of combination therapy1. However, real-world (RW) data on UPA in clinical practice are limited. This analysis presents 6-month data on the effectiveness and safety of UPA in pts with moderate/severe RA in RW clinical practice.
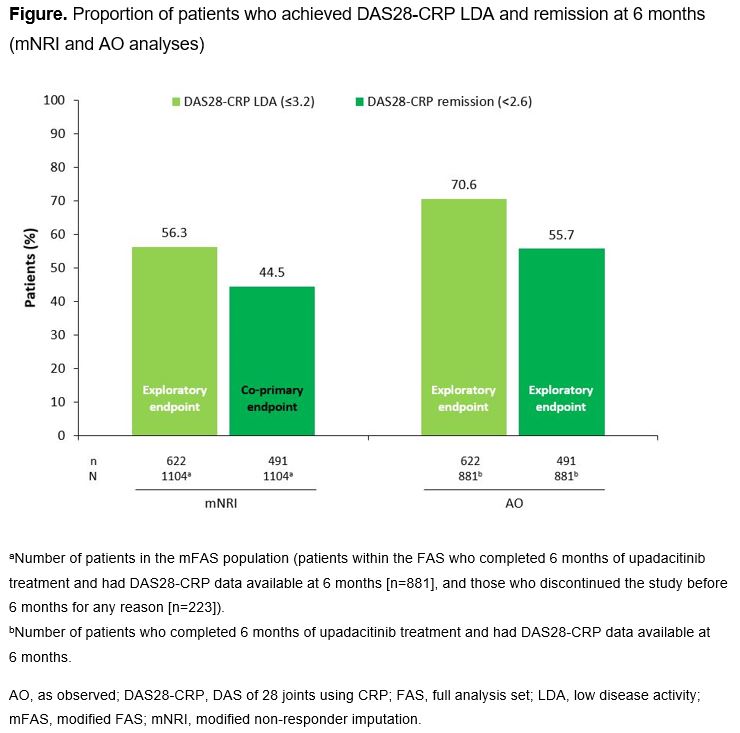
Methods: UPHOLD (NCT04497597) is an ongoing, international, observational cohort study of UPA‑naïve adults (aged ≥18 years) with moderate/severe RA, who received UPA 15 mg once daily (QD) as per the product label, with the decision to initiate UPA made before study enrollment. This 6-month interim analysis includes data reported between the study start date of Oct 16, 2020 and data cutoff of Apr 20, 2023. The co-primary endpoints are: 1) proportion of pts achieving DAS28-CRP remission (< 2.6) at 6 months, and 2) proportion of pts achieving DAS28-CRP remission at 6 months who continue to receive UPA and maintain remission (or have a ≤0.6-point increase in DAS28-CRP) at 12 months; only the first of these is reported here. Secondary and exploratory efficacy endpoints reported include proportion of pts achieving DAS28-CRP low disease activity (LDA; ≤3.2) at 6 months and change from baseline to 6 months in pt-reported outcomes (PROs). Safety (reported as % of pts with adverse events [AEs]) and PROs (as observed [AO] data) were assessed in the full analysis set (FAS; all pts who received ≥1 UPA dose). DAS28-CRP remission and LDA were analyzed using modified non-responder imputation (mNRI; discontinuations before 6 months for any reason were treated as non-responders) in a modified FAS (mFAS; all pts within the FAS who completed 6 months of UPA treatment and had DAS28-CRP data available, and pts who discontinued the study before 6 months for any reason). Remission and LDA at 6 months were also analyzed AO in the mFAS population.
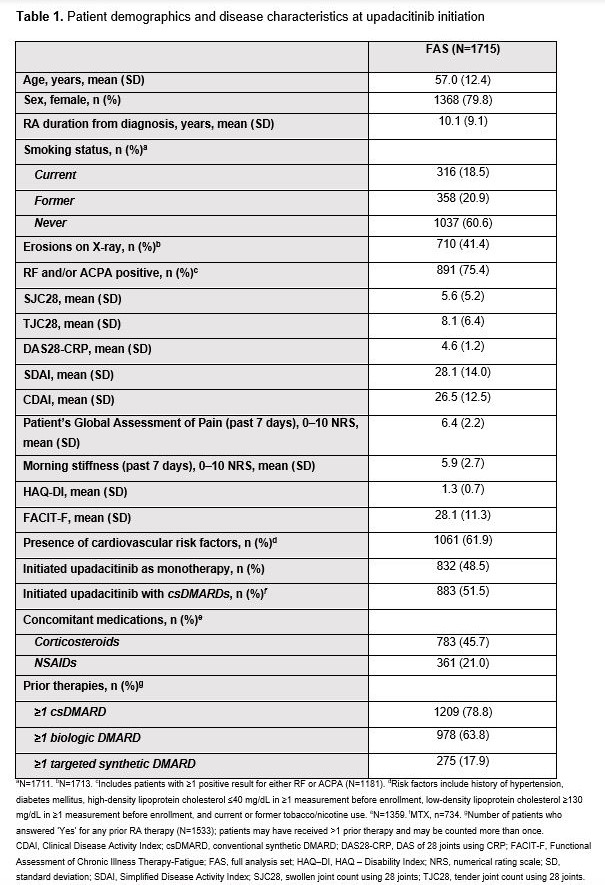
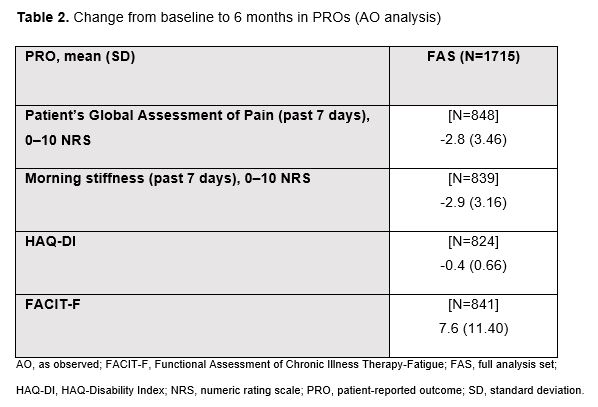
Results: Of the 1732 pts enrolled, 1715 received ≥1 UPA dose (FAS), of whom 223 (13%) prematurely discontinued the study before 6 months. The most common primary reasons for discontinuation were AEs and lack of efficacy (n=72 [4.2%] each). Pt demographics and disease characteristics at UPA initiation are summarized in Table 1. Of the 1104 pts in the mFAS, 44.5% (mNRI) and 55.7% (AO) achieved DAS28-CRP remission at 6 months, and 56.3% (mNRI) and 70.6% (AO) achieved DAS28-CRP LDA at 6 months (Figure). Improvements from baseline to 6 months were observed across PROs (Table 2). AEs reported were: any AE (36.4% of pts), serious AEs (4.5%), serious infection (1.7%; most commonly COVID-19), herpes zoster (2.5%), creatine phosphokinase elevation (0.5%), malignancy excluding non-melanoma skin cancer (NMSC; 0.3%), NMSC (0.4%), venous thromboembolic events (0.4%), and deaths (0.3%). No major adverse cardiovascular events were reported.
Conclusion: The results from UPHOLD suggest that UPA 15 mg QD is effective for the treatment of moderate/severe RAin RW practice, with almost half of pts achieving remission at 6 months. The benefit–risk profile of UPA remains favorable in the RW, consistent with that observed in Phase 3 clinical trials1.
Reference:
1. Conaghan PG, et al. Drug Saf 2021;44:515–30
To cite this abstract in AMA style:
Östör A, Feist E, Sidiropoulos P, Avouac J, Rebella M, Namas R, Frediani B, Hsieh S, Gao T, Khan N, Strengholt S, Lagunes-Galindo I, Attar S. Real-World Effectiveness of Upadacitinib in Patients with Moderate/Severe Rheumatoid Arthritis: 6-Month Data from the Observational UPHOLD Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/real-world-effectiveness-of-upadacitinib-in-patients-with-moderate-severe-rheumatoid-arthritis-6-month-data-from-the-observational-uphold-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-effectiveness-of-upadacitinib-in-patients-with-moderate-severe-rheumatoid-arthritis-6-month-data-from-the-observational-uphold-study/