Session Information
Date: Sunday, October 26, 2025
Title: (0233–0279) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Eosinophilic gastrointestinal disorders (EGIDs) are a group of disorders characterized by marked eosinophilic infiltration of the gastrointestinal (GI) tract resulting in organ dysfunction and onset of GI symptoms. They may present isolated or as part of systemic diseases including eosinophilic granulomatosis with polyangiitis (EGPA) and hypereosinophilic syndrome (HES). Mepolizumab (MEPO), an anti-interleukin 5 monoclonal antibody, is approved for both of these conditions; however, data are limited regarding its role in managing GI involvement. The aim of this study is to retrospectively evaluate the efficacy of MEPO on EGPA and HES-related GI involvement.
Methods: This was a retrospective cohort study conducted among centres referring to the European EGPA Study Group. Inclusion criteria: age > 18 years; diagnosis of EGPA ([1], [2]) or HES ([3]); GI manifestations confirmed by histology or imaging; start of MEPO any dosage (ie 100 mg/month sc, 300 mg/month sc, 750 mg/month iv) for uncontrolled GI manifestations. Data were collected from disease onset until MEPO start (t0), at 1 (t1), 3 (t3), 6 (t6), 12 (t12), and 24 (t24) months, as well as during flares (defined as exacerbations of GI symptoms requiring therapeutic escalation). Outcomes included symptom improvement, absolute eosinophil count (AEC) reduction, flare occurrence, and corticosteroid (CS)-sparing. Data are presented as n (%) or median (IQR). Wilcoxon or McNemar tests were used as appropriate. Flare rates (flares/patient-month) pre- and post-MEPO were compared using incidence rate ratio (IRR) with 95% CI (p< .05).
Results: Forty-eight patients were included [23 EGPA, 25 HES; median age at onset: 38 (30-51) years; median age at diagnosis: 43 (33-55) years; median diagnostic delay: 12 (3-44) months]. At baseline, all the patients had active GI involvement, with a median peak AEC of 3857 (1593-9743) cells/μL. Overall, 46 patients (96%) had a history of CS use prior to MEPO. Median time from diagnosis to MEPO start was 5 (1-49) months (Table 1). Median AEC significantly decreased by t1 [t0: 805 (482-1805) cells/μL; t1: 115 (60–223); p< .001] and remained low through t24 [70 (23–108) cells/μL; p< .001]. By t24, a significant decrease in the proportion of patients with active GI symptoms (65% to 12%, p< .001) and oral CS (OCS) use (85% to 46%, p=.001) were seen. Median prednisone equivalent daily dose decreased from 11.3 (5-25) mg/day at t0 to 0 (0-2.5) mg/day at t24 (p< .001) (Table 2). During follow up, 8 patients experienced a GI relapse [4 EGPA, 4 HES; median time to event: 9 (4-23) months]. All were on a stable MEPO dose, 3 were on OCS (2 tapering) and 1 on MMF. The AEC increased AEC in only 1 case. All flares were managed by adjusting CS therapy; additionally, MEPO dose was increased in 2 cases and immunosuppressive therapy was added in 2 others (Table 3). The flare rate decreased from 0.021 to 0.0049 flares per patient-month after MEPO initiation (IRR 0.23, 95% CI 0.11–0.49, p=.0001), representing a 77% reduction.
Conclusion: MEPO effectively controlled GI symptoms and reduced CS use in the majority of EGPA and HES patients. References: [1] Grayson et al., Ann Rheum Dis 2022;81:309–14 [2] Wechsler et al., N Engl J Med 2017;376:1921–32 [3] Valent et al., Allergy 2023;78:47–59
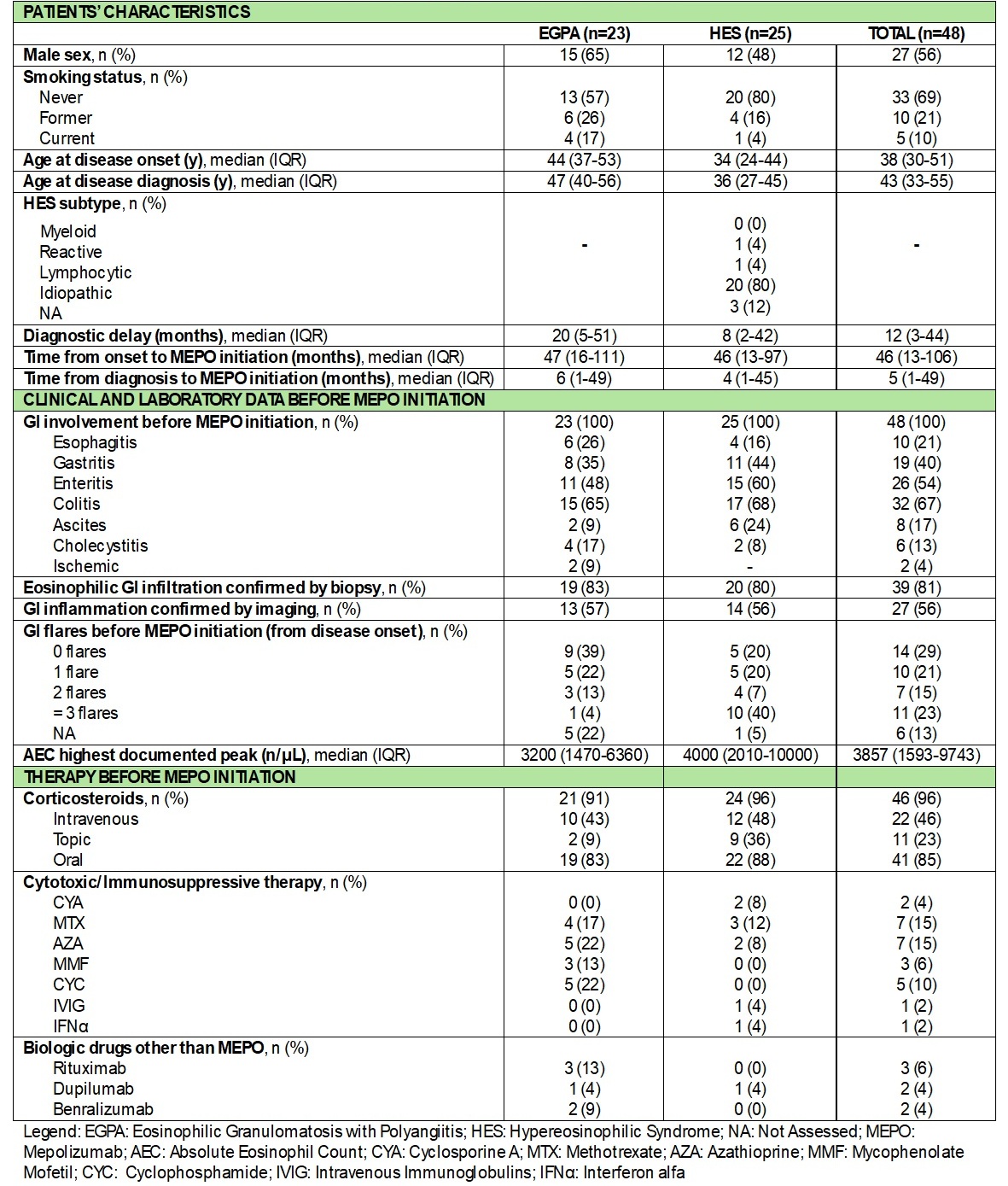 Table 1. Patients’ characteristics and clinical, laboratory, and treatment data before MEPO
Table 1. Patients’ characteristics and clinical, laboratory, and treatment data before MEPO
.jpg) Table 2. Clinical, laboratory, and treatment data collected at MEPO initiation and during follow up
Table 2. Clinical, laboratory, and treatment data collected at MEPO initiation and during follow up
.jpg) Table 3. Summary of disease flare characteristics and related treatments
Table 3. Summary of disease flare characteristics and related treatments
To cite this abstract in AMA style:
Gatti A, Regola F, Fontana G, Mora J, Piga M, Moroncini G, Acquaviva R, Carlucci P, Noviello S, Vacca A, Delvino P, Heffler E, Nappi E, Moll J, Schroeder J, Losappio L, Davanzo F, Padoan R, Cameli P, Conticini E, Pacini G, Berti A, Vrola L, Tomietto P, Fazzi B, treppo e, Quartuccio L, Cohen Tervaert J, Arnold S, Lamprecht P, Roufosse F, Cavazzana I, Franceschini F, Emmi G, Toniati P. Real-World Effectiveness of Mepolizumab on Gastrointestinal Involvement in Eosinophilic Granulomatosis with Polyangiitis and Hypereosinophilic Syndrome: A Multicenter Retrospective Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-effectiveness-of-mepolizumab-on-gastrointestinal-involvement-in-eosinophilic-granulomatosis-with-polyangiitis-and-hypereosinophilic-syndrome-a-multicenter-retrospective-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-effectiveness-of-mepolizumab-on-gastrointestinal-involvement-in-eosinophilic-granulomatosis-with-polyangiitis-and-hypereosinophilic-syndrome-a-multicenter-retrospective-study/
