Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Interstitial lung disease (ILD) is a critical complication of rheumatoid arthritis (RA). Abatacept and rituximab are the preferred disease-modifying antirheumatic drugs (DMARDs) for RA-ILD. However, progression of ILD despite its use is not uncommon. A subgroup analysis of the INBUILD trial has shown a slower decline in forced vital capacity (FVC) in patients with progressive fibrosing autoimmune disease-related ILD with nintedanib (NINTE) [Arthritis Rheumatol 2022;74(6):1039-47]. However, data on antifibrotics use for RA-ILD in clinical practice (CP) are scarce. Our aim was: a) To assess the efficacy of antifibrotic drugs, NINTE and pirfenidone (PIRFE), in Spanish RA-ILD patients with a progressive phenotype in CP; b) To compare the profile of CP RA-ILD patients with those included in the INBUILD trial.
Methods: National multicenter study of RA-ILD patients to whom NINTE or PIRFE were added due to progressive fibrosing ILD. Demographic and clinical variables were collected from all patients. These features were compared with those of RA-ILD patients included in the INBUILD trial (n=89, 42 NINTE and 47 placebo). Forced vital capacity (FVC) evolution was the primary endpoint. Results are expressed as percentage, mean±SD or median [IQR].
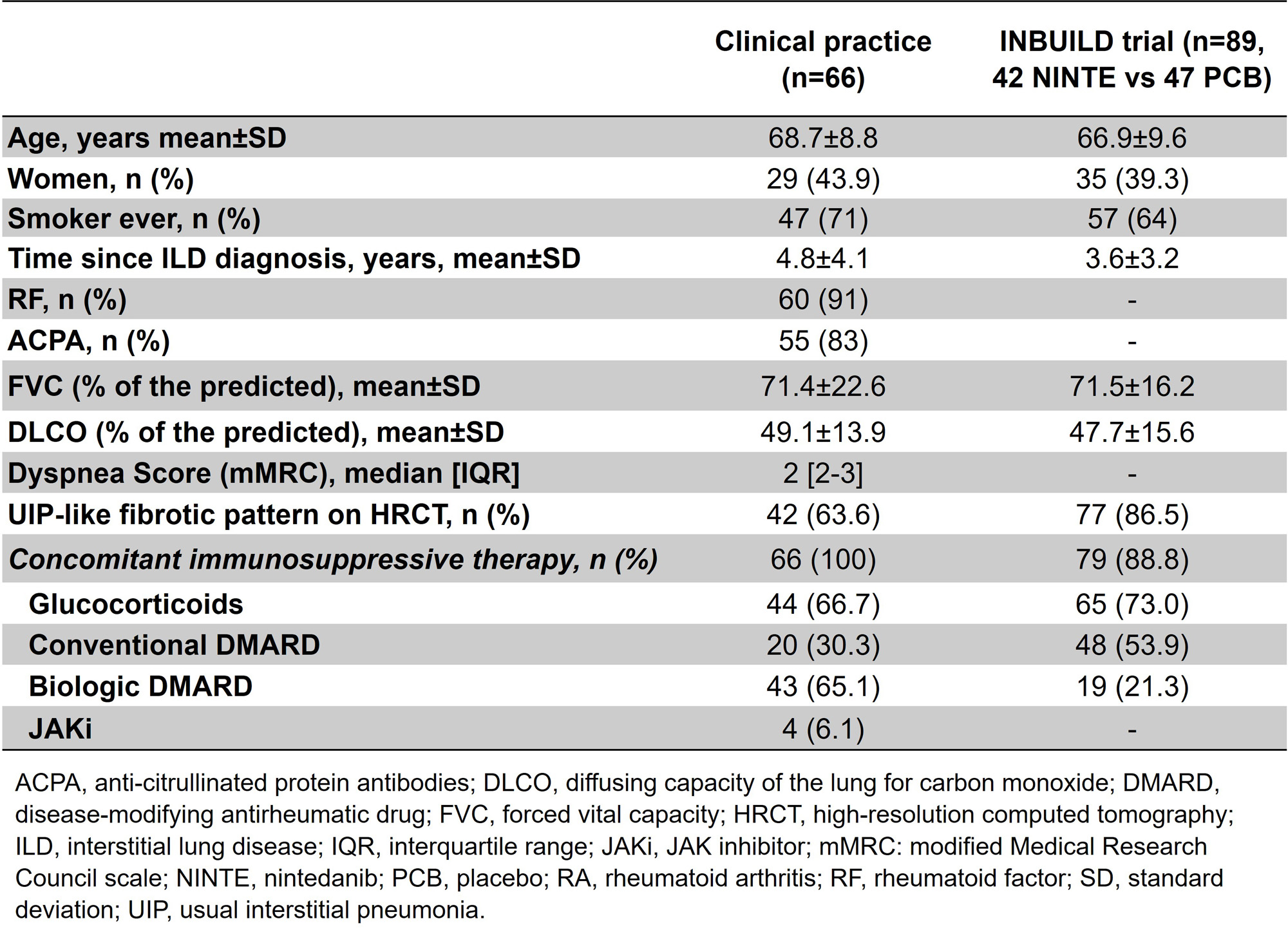
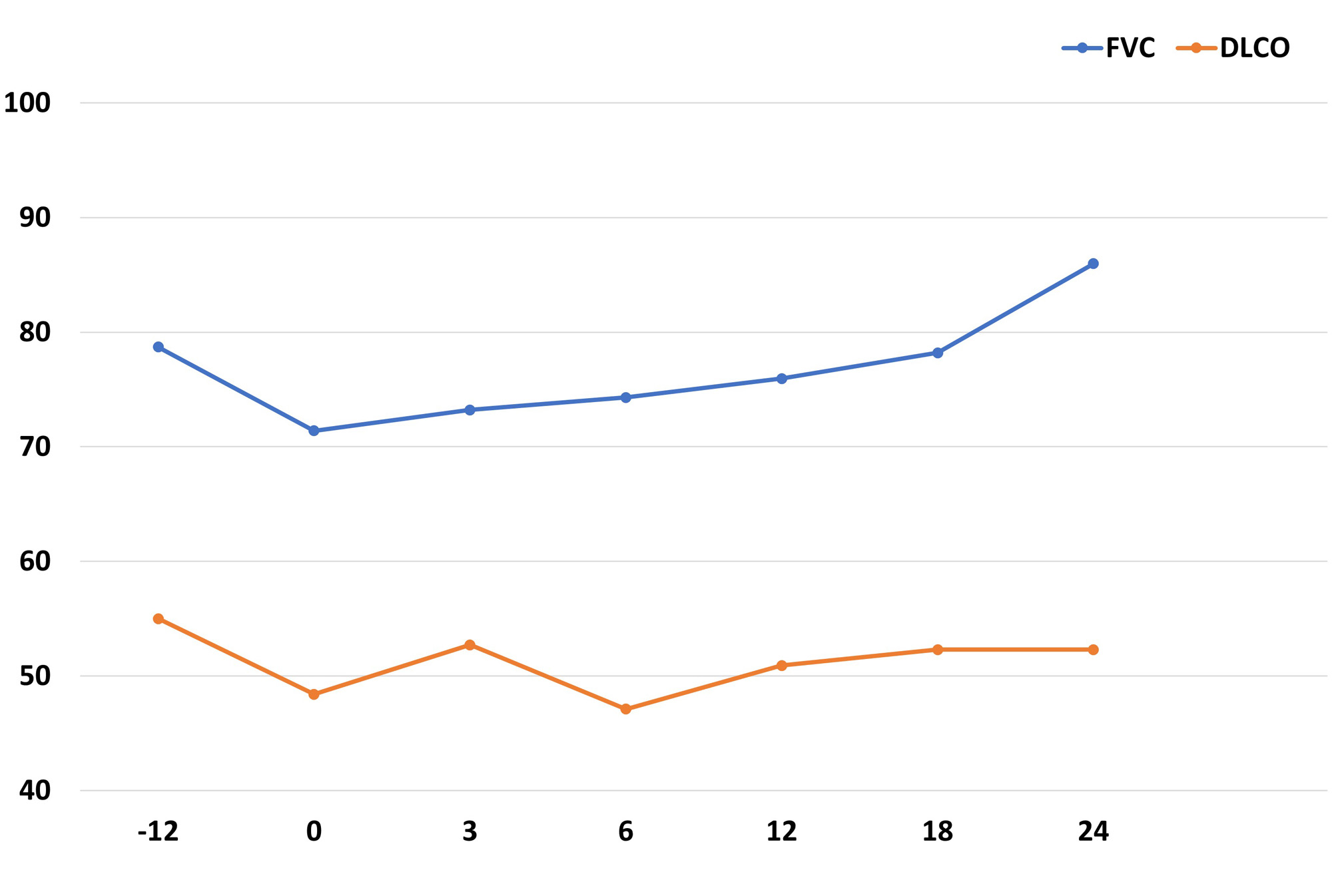
Results: A total of 73 patients (24 women/49 men) from CP were collected, mean age of 69±9 years. The median [IQR] ILD duration up to antifibrotic initiation was of 56.5 [21.5-83] months. NINTE was the most widely used antifibrotic (n=66), combined with immunosuppressive treatment in all cases: corticosteroids in 44, cDMARD in 20 (mycophenolate mofetil=8, leflunomide=6, methotrexate=3, hydroxychloroquine=2, azathioprine=1), bDMARD in 43 (abatacept=31, rituximab=10, anti-IL6R=2) and/or JAKi in 4 (baricitinib=2, tofacitinib=1, filgotinib=1). Mean FVC one year before NINTE start was 80±21 (% pred.), whilst mean baseline FVC was 71±23 (% pred.). Table 1 shows the comparison of baseline characteristics of RA-ILD patients treated with NINTE in CP and in the INBUILD trial. The evolution of FVC and diffusing capacity of the lungs for carbon monoxide (DLCO) in our patients with NINTE from the previous year of antifibrotic initiation is shown in Figure 1. After a median follow-up of 15.5 [3.5-23.5] months, no significant decline in mean FVC or DLCO values was observed. In addition, 86% of the patients presented stabilization or improvement of dyspnea. NINTE was withdrawn in 14 patients due to: gastrointestinal adverse events (11), death (1), hemorrhage risk (1), and stabilization (1). PIRFE was administered to 7 patients (4 men), combined with abatacept in 3 patients, leflunomide in 1, and methotrexate in 1. Mean baseline FVC and DLCO were 69±22 and 49±14 (% pred.), respectively. As with NINTE, a stability in the evolution of lung function was observed. PIRFE was withdrawn in 4 patients due to: gastrointestinal adverse events (2), inefficacy (1), and stabilization (1).
Conclusion: Antifibrotics, especially NINTE, seem to slow ILD progression in patients with RA-ILD. In CP, our patients are treated belatedly in the evolution of the disease, but results are satisfactory. Combination of antifibrotics and DMARDs is feasible and safe.
To cite this abstract in AMA style:
Atienza-Mateo B, Serrano-Combarro A, Martin-Lopez M, Castañeda S, Melero-Gonzalez R, Loarce-Martos J, Mena Vazquez N, carrasco-Cubero C, Diez-Morrondo C, Castro-Corredor D, Vazquez-Rodriguez T, Garcia-Valle a, Bonilla G, Rodriguez-lopez M, Brana Abascal I, ROJAS HERRERA S, Sarmiento-Monroy J, Andujar-Brazal P, De Dios J, Gonzalez-Montagut Gomez C, Ordonez-Palau S, Brandy-Garcia A, Lozano F, Lopez-Lasanta M, Campos Fernández C, Garijo Bufort M, Casafont-Sole I, Goercke C, Iñiguez C, Ortiz-Sanjuán F, Giner-Serret E, Flores Robles B, Moreno M, Cervantes-Perez E, Ferrer D, Blanco R, Collaborative Group Members O. Real World Data on Antifibrotics in Rheumatoid Arthritis-Interstitial Lung Disease. National Multicenter Study of 73 Patients [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/real-world-data-on-antifibrotics-in-rheumatoid-arthritis-interstitial-lung-disease-national-multicenter-study-of-73-patients/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-data-on-antifibrotics-in-rheumatoid-arthritis-interstitial-lung-disease-national-multicenter-study-of-73-patients/