Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA and was approved under Taiwan’s National Health Insurance (NHI) reimbursement system in December 2014. Here, we report final data from the XTRA registry on the long-term effectiveness, safety, and persistence/adherence of tofacitinib and TNF inhibitors (TNFi) in Taiwanese patients (pts) with RA.
Methods: This 5-year prospective, multicenter, observational study consisted of a 24-month enrollment period and ≥ 36-month follow-up period (August 2016–May 2021). Pts received standard care for RA according to the treating rheumatologists and fulfillment of the NHI reimbursement criteria (DAS28 in 28 joints, with ESR [DAS28-ESR] > 5.1; failure of ≥ 2 DMARDs, including MTX; and no tuberculosis). Demographics and baseline disease characteristics were compared between groups using a chi-squared and two-sample t-tests for categorical and continuous variables, respectively, with no adjustment for multiplicity. DAS28-ESR, Clinical Disease Activity Index (CDAI), HAQ-Disability Index (HAQ-DI), and Work Productivity and Activity Impairment Index-RA (WPAI-RA) were assessed at baseline and 24-week intervals thereafter; differences between groups were compared using mixed logistic regression models. Propensity scores were used to adjust for confounding by treatment group. Safety was assessed throughout the study. Persistence (total number of days between first and last refill date plus days’ supply of last refill) and adherence (proportion of days covered [PDC]) were assessed for the entire study period.
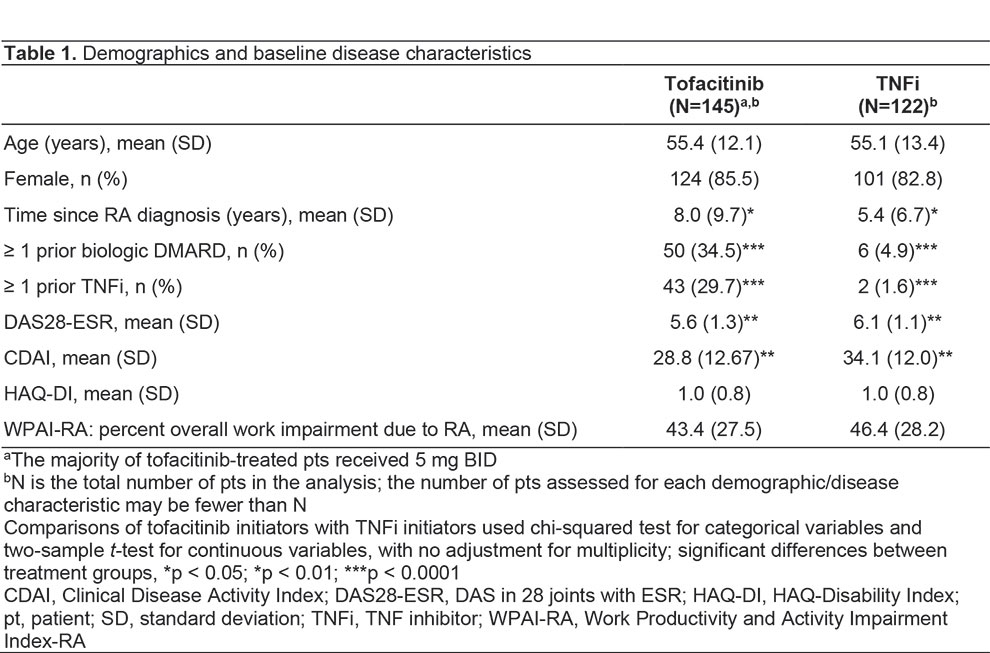
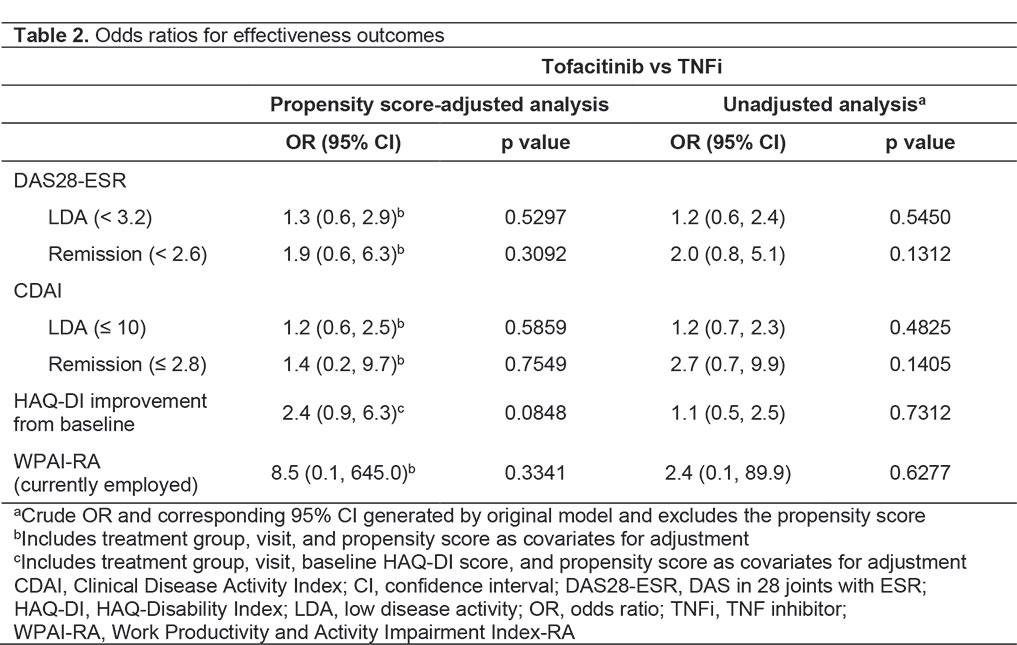
Results: A total of 267 pts were enrolled (tofacitinib, n=145; TNFi, n=122). Demographics were generally similar between groups, with significant differences reported in some baseline disease characteristics (Table 1). No significant differences in effectiveness outcomes for tofacitinib vs TNFi were reported before or after propensity score adjustment (Table 2). Rates of adverse events (AEs), serious AEs (SAEs), and deaths were similar across treatment groups (Table 3). Most AEs were mild or moderate in severity. Pneumonia (1.9%), herpes zoster (1.5%), and urinary tract infection (1.5%) were the most frequent SAEs in the study population. Among AEs of special interest in tofacitinib and TNFi pts, serious infections occurred in 13.1% and 8.2% of pts; MACE in 0 pts and 0.8% of pts; and malignancy in 2.1% and 0.8% of pts, respectively (Table 3). No instances of venous thromboembolism were reported. For patients receiving tofacitinib or TNFi, respectively, mean (standard deviation [SD]) persistence was 996.2 (498.3) days and 986.0 (537.2) days, and mean (SD) PDC was 80.5% (30.3%) and 78.4% (33.2%).
Conclusion: In this analysis of real-world data, tofacitinib effectiveness, safety, and persistence/adherence were generally similar to TNFi in pts with RA. Safety outcomes were consistent with the known safety profile of tofacitinib and TNFi. Limitations included small sample size and residual confounding due to the observational nature of the study.
Study sponsored by Pfizer. Medical writing support was provided by J Maxwell, CMC Connect, and funded by Pfizer Inc.
To cite this abstract in AMA style:
Hsieh S, Chen W, Hu J, Sung W, Tsai W, Chen H, Huang C, Kuo E, Wee J, Woolcott J, Chen Y. Real-world Data of Tofacitinib versus Tumor Necrosis Factor Inhibitors in Taiwanese Patients with Rheumatoid Arthritis from a Drug-based Registry [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/real-world-data-of-tofacitinib-versus-tumor-necrosis-factor-inhibitors-in-taiwanese-patients-with-rheumatoid-arthritis-from-a-drug-based-registry/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-data-of-tofacitinib-versus-tumor-necrosis-factor-inhibitors-in-taiwanese-patients-with-rheumatoid-arthritis-from-a-drug-based-registry/