Session Information
Date: Tuesday, November 19, 2024
Title: Abstracts: RA – Treatment II: Refining Use of Established Therapies
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: For patients with rheumatoid arthritis (RA), current ACR guidelines recommend evaluating treatment response within 3 months of initiating a new therapy. In patients who do not achieve an early treatment response, the likelihood of a future treatment response while on the same therapy is not well understood. The aim of this analysis was to evaluate the subsequent treatment response among patients with RA who initiated and remained on a 1st-line tumor necrosis factor inhibitor (TNFi) after achieving or not achieving an initial treatment response.
Methods: This analysis used linked administrative health care claims and electronic medical record data from the OM1® Real-World Data Cloud. Adults with RA initiating a 1st-line TNFi between January 1, 2016, and March 31, 2023, who were in moderate/high disease activity (MDA/HDA) at TNFi initiation (Clinical Disease Activity Index [CDAI] >10) and remained on 1st-line TNFi for ≥12 months were included. Follow-up CDAI was assessed from months 3 (±30 days) to 12 (±45 days) after TNFi initiation. Treatment response in the primary analysis was defined as achievement of CDAI low disease activity (LDA) or remission (CDAI ≤10). In the secondary analysis, treatment response was defined as achievement of CDAI minimal clinically important difference (MCID; defined as ≥6-point improvement from baseline CDAI in MDA and ≥12-point improvement from baseline CDAI in HDA). Proportions of patients with a treatment response at 3 months were reported. Following the 3-month visit, the subsequent treatment response at 12 months and at all visits with an available CDAI from months 3 to 12 were evaluated.
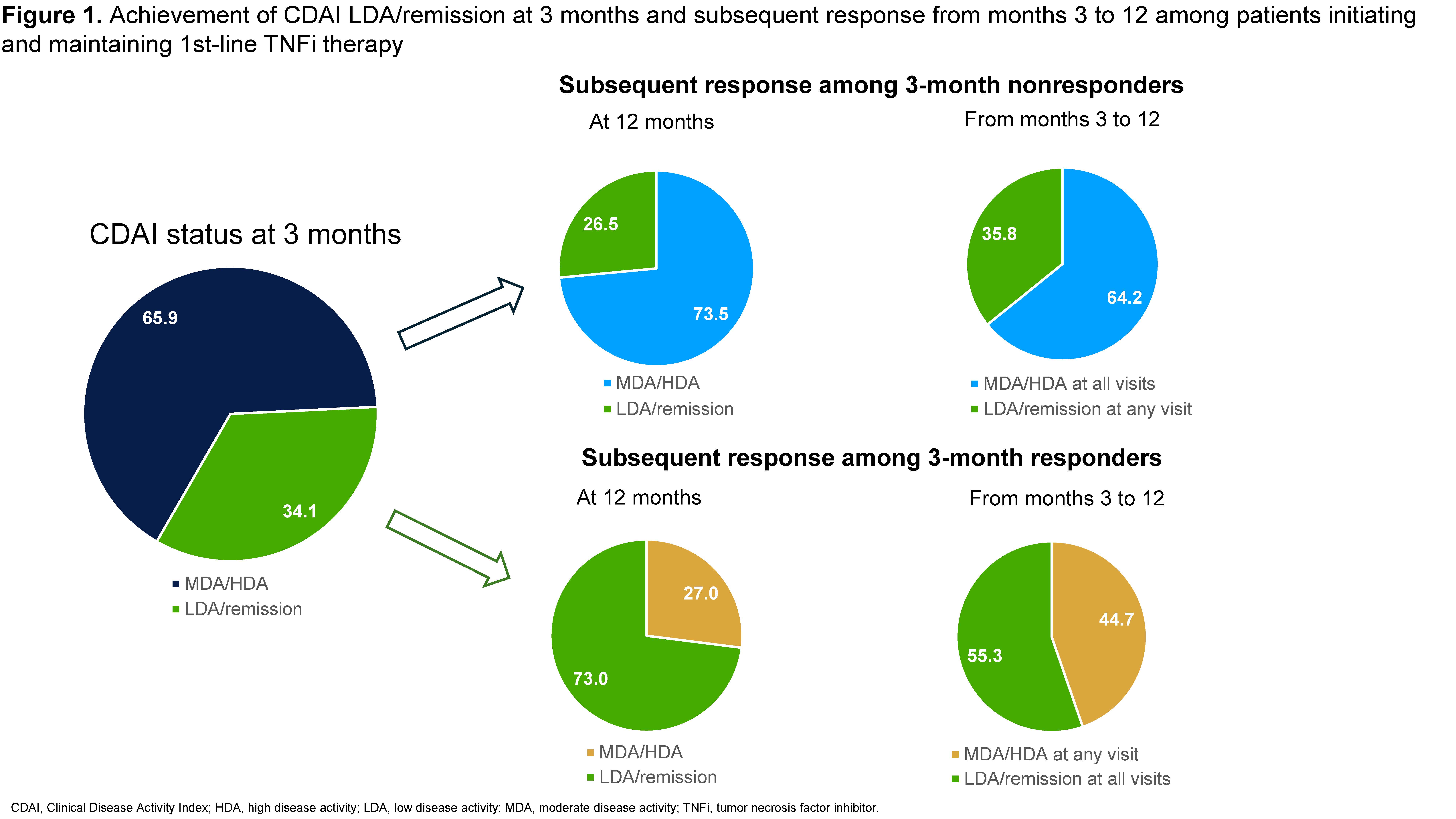
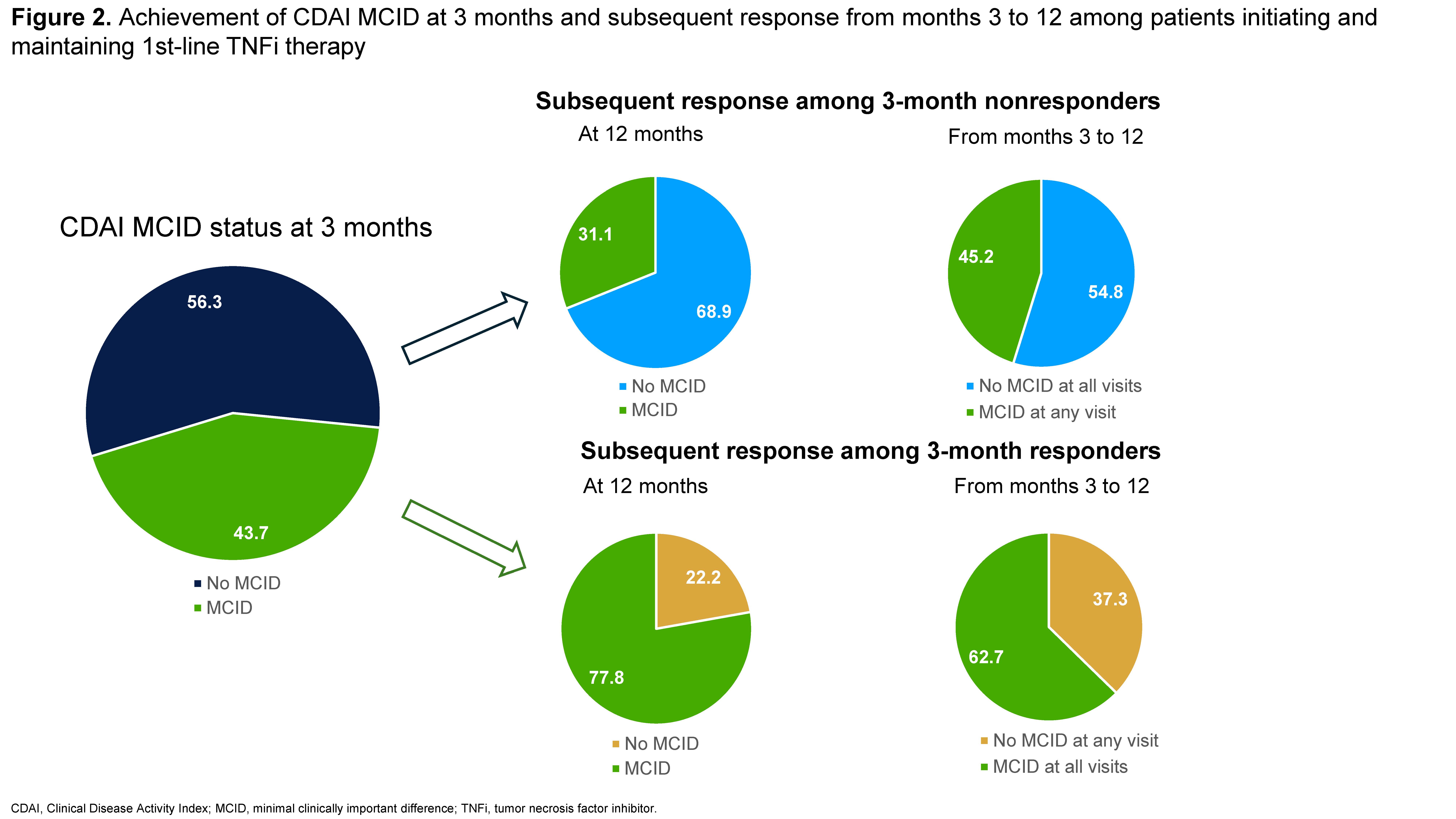
Results: Data from 1215 patients were analyzed, the mean age (standard deviation [SD]) was 59.2±13.3 years and 77.1% were female. In the primary analysis, 65.9% (n=801) of patients remained in MDA/HDA while 34.1% (n=414) were in LDA or remission at 3 months after 1st-line TNFi initiation (Figure 1). Among patients in MDA/HDA at 3 months, 73.5% were in MDA/HDA at 12 months and 64.2% were in MDA/HDA at all follow-up visits from months 3 to 12 (mean number of follow-up visits with CDAI=2.7). Among patients in LDA/remission at 3 months, 73.0% were in LDA/remission at 12 months, but only 55.3% were in LDA/remission at all follow-up visits from months 3 to 12 (mean number of follow-up visits with CDAI=2.5). In the secondary analysis, 43.7% (n=531) achieved a CDAI MCID response at 3 months while 56.3% (n=684) did not (Figure 2). Of patients not responding at 3 months, 68.9% did not achieve CDAI MCID at 12 months and 54.8% did not achieve CDAI MCID at any visit from months 3 to 12. Among 3-month responders, 77.8% achieved MCID at 12 months and 62.7% achieved MCID at all visits from months 3 to 12.
Conclusion: Among patients with RA initiating a 1st-line TNFi, the majority of those who did not achieve a treatment response at 3 months also did not achieve a subsequent response from months 3 through 12. Further, approximately 1 in 4 patients achieving LDA/remission at 3 months were no longer in LDA/remission at month 12. These findings may be useful to inform clinical decision-making on treatment continuation after initial nonresponse in patients with RA initiating 1st-line TNFi therapy.
To cite this abstract in AMA style:
Charles-Schoeman c, Zueger P, Blondell E, Fang S, Peng Y, Jain M, Tesser J. Real-World Analysis of Initial Clinical Response and Future Outcomes Among Patients with Rheumatoid Arthritis Initiating and Remaining on a 1st-Line Tumor Necrosis Factor Inhibitor in the United States [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/real-world-analysis-of-initial-clinical-response-and-future-outcomes-among-patients-with-rheumatoid-arthritis-initiating-and-remaining-on-a-1st-line-tumor-necrosis-factor-inhibitor-in-the-united-state/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-analysis-of-initial-clinical-response-and-future-outcomes-among-patients-with-rheumatoid-arthritis-initiating-and-remaining-on-a-1st-line-tumor-necrosis-factor-inhibitor-in-the-united-state/