Session Information
Date: Monday, November 9, 2015
Title: Systemic Lupus Erythematosus - Clinical Aspects and Treatment Poster Session II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Randomized controlled trials (RCTs) are the gold standard for assessing
treatment efficacy. However, due to their often strict
design, generalization of research results to real populations may be
problematic. Our objective was to estimate the proportion of our lupus nephritis
(LN) patients who would have been ineligible to participate in RCTs, as an
indicator of how well LN RCT findings could be transferred to our clinical
practice.
Methods: A systematic
literature search until May 2015 was performed using PubMed, Medline, EMBASE
and Cochrane databases to identify full text English publications reporting RCTs
on LN. We focused on trial inclusion and exclusion criteria related to lupus. The
criteria were classified into 3 broad categories: (1) severe disease (low GFR, high
serum creatinine); (2) mild disease (low level proteinuria, serum albumin, relatively
preserved GFR) and (3) prohibited immunosuppressive drugs used at time of LN
onset. LN patients with biopsy proven proliferative (class III, IV) or
membranous (class V) LN, diagnosed 1995-2013, were identified from our
database; their baseline characteristics were compared with each RCT’s entry
criteria for eligibility.
Results: We
identified 137 patients with a biopsy proven diagnosis of active LN, with mean
age of 33.6 years (± 11.5 SD) at LN onset. 85.4% (n=117) were female, 50% were
Black, 34% were Asian and 16% were White Caucasian. 81% had proliferative
(n=111, 30% class III, 51% class IV, with or without membranous component),
whilst 19% had pure membranous LN (n=26). Baseline clinical activity showed mean
proteinuria of 5.5 g/24hr (± 5.1 SD), serum albumin of 27.3 g/L (± 7.4 SD), and
serum creatinine of 159 μmol/L
(± 166 SD). Whilst 33% (n=45) had a normal GFR (>90 ml/min/1.73m2),
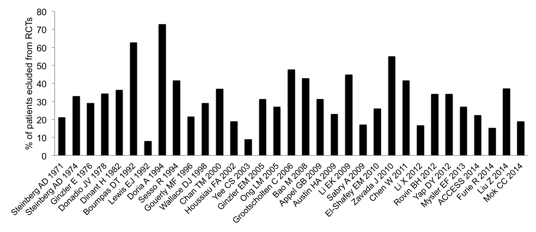
18% had a GFR <30 ml/min/1.73m2. 33 RCTs were selected from the
databases, of which only 4 published the number of patients screened, and only
two disclosed the factors leading to non-randomization. Overall, on average, 32%
of our LN patients were not eligible to enter the RCTs (range 8-73%) (Figure).
26 RCTs (79%) excluded patients with category 1 (severe disease) which would
have rejected up to 61% of our patients from trial inclusion; 20 RCTs (63%) excluded
patients with category 2 (mild disease) which would have omitted up to 44% of
our cohort, and 22 trials (67%) excluded patients with category 3 (prior use of
selected immunosuppressive drugs) resulting in ineligibility of up to 16% of
our cohort.
Conclusion: Nearly 1/3
of our newly diagnosed active LN population would have been excluded from RCTs
by design, due to factors directly related to their renal disease. Clinicians
should be aware that trial samples may not adequately reflect their LN population
and therefore research results may not be generalizable to all LN patients in clinical
practice. Our study highlights the need for more pragmatic trials designed for
those with milder or more severe LN.
To cite this abstract in AMA style:
Pakozdi A, Rajakariar R, Yaqoob MM, Pyne D. Real Life Implementation of Lupus Nephritis Randomized Controlled Trials [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/real-life-implementation-of-lupus-nephritis-randomized-controlled-trials/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-life-implementation-of-lupus-nephritis-randomized-controlled-trials/